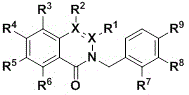
Preparation method of polysubstituted 2-benzyl-1-isoquinolone compound
A technology for isoquinolinones and compounds, which is applied in the field of preparation of multi-substituted 2-benzyl-1-isoquinolinone compounds, can solve the problems of complex synthesis steps, complex operations, and many steps, and achieve easy separation and purification , mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, preparation 2-benzyl-1 (2H)-isoquinolinone compound
[0018] The reaction formula is as follows:
[0019] Formula (Ia)
[0020] The specific preparation method is: without special protection, add magneton, isoquinoline (51.6mg, 0.4mmol), and then add iodine (1mg, 1mol%), 5.0M-6.0M to a clean single-necked flask under air Concentration of tert-butyl hydroperoxide (360mg, 2mmol) in the organic phase was added, and toluene (728.8mg, 8mmol) was added. Toluene was used as a reactant and as a solvent. After 12 hours of reaction at 120°C, TLC analysis showed that the raw materials were different. Quinoline is completely consumed. The reaction was quenched by stopping the heating. No extraction, direct wet loading, 200-300 mesh silica gel column chromatography, eluting with a mixed solvent of ethyl acetate and petroleum ether (1:8). 79.0 mg of the compound represented by the structural formula of formula Ia was isolated, with a yield of 84%;
[0021] The pr...
Embodiment 2
[0026] Embodiment 2, the 2-benzyl-1 (2H)-isoquinolinone compound shown in preparation formula Ia
[0027] The reaction formula is as follows:
[0028] Formula (Ia)
[0029] The specific preparation method is: without special protection, add magnetons, isoquinoline (51.6mg, 0.4mmol), and then add potassium iodide (99.6mg, 150mol%), 70% mass fraction of tert-butyl hydroperoxide (205mg, 1.6mmol) in the water phase was added toluene (364.4mg, 4mmol), and toluene was used as both a reactant and a solvent. After reacting at 150°C for 0.5 hours, TLC analysis showed that the raw material isoquinone The phylloline is completely consumed. The reaction was quenched by stopping the heating. No extraction, direct wet loading, 200-300 mesh silica gel column chromatography, eluting with a mixed solvent of ethyl acetate and petroleum ether (1:8). 48.0 mg of the compound represented by the structural formula of formula Ia was isolated, with a yield of 51%;
[0030] Compound structure an...
Embodiment 3
[0031] Embodiment 3, the 2-benzyl-1 (2H)-isoquinolinone compound shown in preparation formula Ia
[0032] The reaction formula is as follows:
[0033] Formula (Ia)
[0034]The specific preparation method is: without special protection, add magneton, isoquinoline (51.6mg, 0.4mmol), and then add cuprous iodide (7.6mg, 10mol%), 70% The mass fraction of tert-butyl hydroperoxide (152mg, 1.2mmol) in the water phase was added toluene (364.4mg, 4mmol). Toluene was used as both a reactant and a solvent. After 36 hours of reaction at 90°C, TLC analysis showed that The raw material isoquinoline is completely consumed. The reaction was quenched by stopping the heating. No extraction, direct wet loading, 200-300 mesh silica gel column chromatography, eluting with a mixed solvent of ethyl acetate and petroleum ether (1:8). 73.3mg of the compound represented by the structural formula of formula Ia was isolated, with a yield of 78%;
[0035] Compound structure analysis and identificati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com