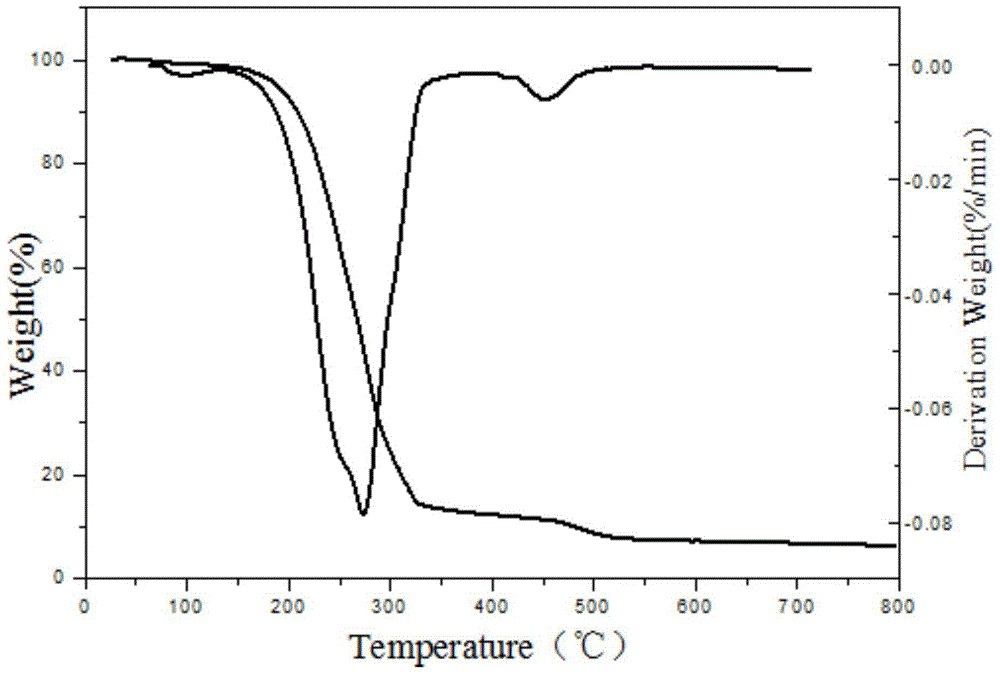
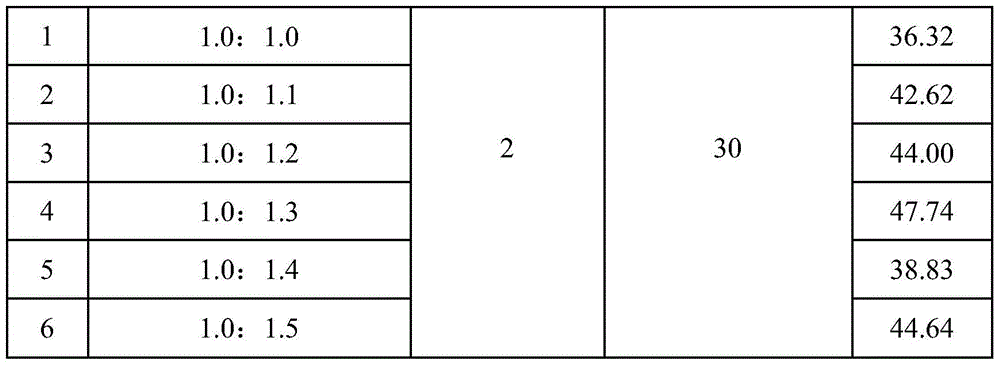
A kind of synthetic method of two (2-carboxyethyl) phosphonic acid
A technology of carboxyethyl and synthetic methods, which is applied in the field of flame retardants, can solve the problems of poor compatibility with polymers, low flame retardant effect, and high toxicity, and achieve good compatibility, short reaction time, and high performance small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The commercially available hypophosphorous acid aqueous solution was distilled under reduced pressure by a rotary evaporator in a water bath at 50° C. to obtain crystalline hypophosphorous acid.
[0030] Under an ice-water bath, 0.28mol (18.4g) of crystalline hypophosphorous acid and 0.28mol (29.71g) of trimethyl orthoformate were added to a three-necked flask, the temperature was raised to 30°C, and the reaction was carried out at this temperature for 2 hours, and then distilled under vacuum to obtain Methanol and methyl formate were removed until a pale yellow solid powder formed.
[0031] At 3°C, 0.84mol (44.52g) of acrylonitrile and 7.7g of catalyst triethylamine were added to the three-necked flask, the temperature was raised to 50°C, and the reaction was carried out for 3h. Then add 0.62mol (62.8g) of concentrated hydrochloric acid (37%), raise the temperature to 90°C, and react for 3h. After the reaction, the solution was cooled, ammonium chloride was removed by...
Embodiment 2
[0033] The commercially available hypophosphorous acid aqueous solution was distilled under reduced pressure by a rotary evaporator in a water bath at 50° C. to obtain crystalline hypophosphorous acid.
[0034] Under an ice-water bath, add 0.28mol (18.4g) of crystalline hypophosphorous acid and 0.31mol (32.90g) of trimethyl orthoformate into a three-necked flask, raise the temperature to 30°C, react at this temperature for 2h, and then distill under vacuum to obtain Methanol and methyl formate were removed until a pale yellow solid powder formed.
[0035] At 3°C, 0.84mol (44.52g) of acrylonitrile and 7.7g of catalyst triethylamine were added to the three-necked flask, the temperature was raised to 50°C, and the reaction was carried out for 3h. Then add 0.62mol (62.8g) of concentrated hydrochloric acid (37%), raise the temperature to 90°C, and react for 3h. After the reaction, the solution was cooled, ammonium chloride was removed by filtration, and then recrystallized in acet...
Embodiment 3
[0037] The commercially available hypophosphorous acid aqueous solution was distilled under reduced pressure by a rotary evaporator in a water bath at 50° C. to obtain crystalline hypophosphorous acid.
[0038] Under an ice-water bath, add 0.28mol (18.4g) of crystalline hypophosphorous acid and 0.34mol (36.08g) of trimethyl orthoformate into a three-necked flask, raise the temperature to 30°C, react at this temperature for 2h, and then distill under vacuum to obtain Methanol and methyl formate were removed until a pale yellow solid powder formed.
[0039] At 3°C, 0.84mol (44.52g) of acrylonitrile and 7.7g of catalyst triethylamine were added to the three-necked flask, the temperature was raised to 50°C, and the reaction was carried out for 3h. Then add 0.62mol (62.8g) of concentrated hydrochloric acid (37%), raise the temperature to 90°C, and react for 3h. After the reaction, the solution was cooled, ammonium chloride was removed by filtration, and recrystallized in acetone / a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com