UV/moisture dual-curing resin and synthesis method thereof
A synthesis method and dual-curing technology, which is applied in the field of light-curing materials, can solve the problems of reduced mechanical properties of the adhesive layer, low photoinitiator initiation efficiency, and difficult curing of the adhesive, so as to achieve good curing effect and stable reaction speed and cured effect, easy to control the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
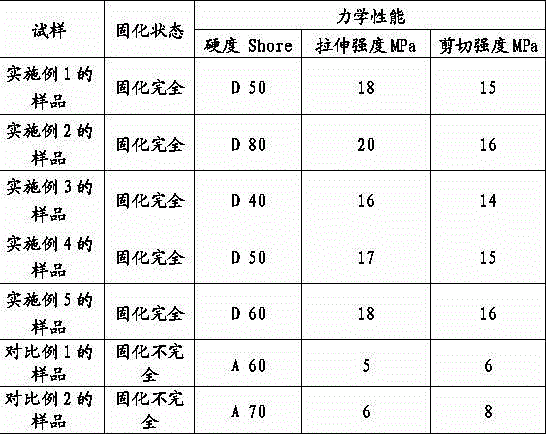
Examples
Embodiment 1
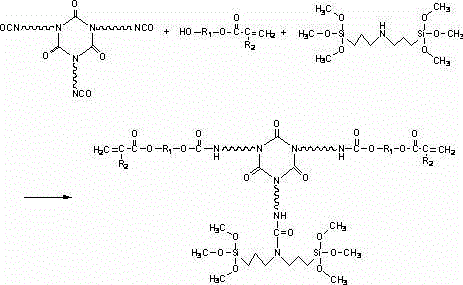
[0026] Add HDI trimer (Bayer, desmodur N3300) 80g, hydroxyethyl acrylate 5g, polymerization inhibitor p-hydroxyanisole 0.1g, catalyst dibutyltin dilaurate 0.01g into a three-necked flask with a thermometer, and heat up At 65 degrees, start to drop 3 g of bis(γ-trimethoxysilylpropyl)amine (cas: 82985-35-1), control the temperature at 75 degrees, and react for 2 hours. Use an infrared spectrometer to analyze the NCO group, and when the NCO peak does not change, stop the heating reaction to obtain a UV / moisture dual-curable resin.
Embodiment 2
[0028] Add HDI trimer (Bayer, desmodur N3300) 110g, hydroxypropyl acrylate 12g, polymerization inhibitor p-hydroxyanisole 0.1g, catalyst dibutyltin dilaurate 0.01g into a three-necked flask with a thermometer, and heat up At 65 degrees, start to drop 6 g of bis(γ-trimethoxysilylpropyl)amine (cas: 82985-35-1), control the temperature at 70 degrees, and react for 3 hours. Use an infrared spectrometer to analyze the NCO group, and when the NCO peak does not change, stop the heating reaction to obtain a UV / moisture dual-curable resin.
Embodiment 3
[0030] Add HDI trimer (Bayer, desmodur N3300) 100g, hydroxyethyl acrylate 8g, polymerization inhibitor p-hydroxyanisole 0.1g, catalyst dibutyltin dilaurate 0.01g into a three-necked flask with a thermometer, and heat up At 65 degrees, start to drop 4 g of bis(γ-trimethoxysilylpropyl)amine (cas: 82985-35-1), control the temperature at 73 degrees, and react for 2 hours. Use an infrared spectrometer to analyze the NCO group, and when the NCO peak does not change, stop the heating reaction to obtain a UV / moisture dual-curable resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com