Histone deacetylases inhibitor
A technology of deacetylase and histone, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
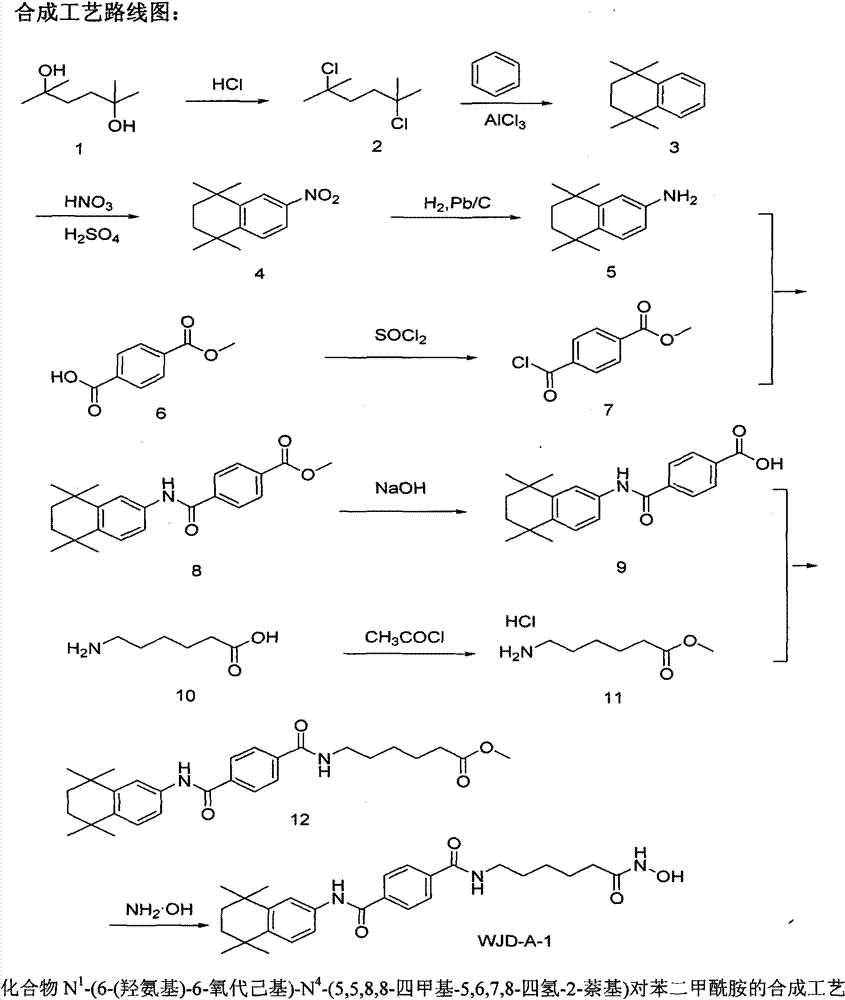
[0010] One, synthesis embodiment
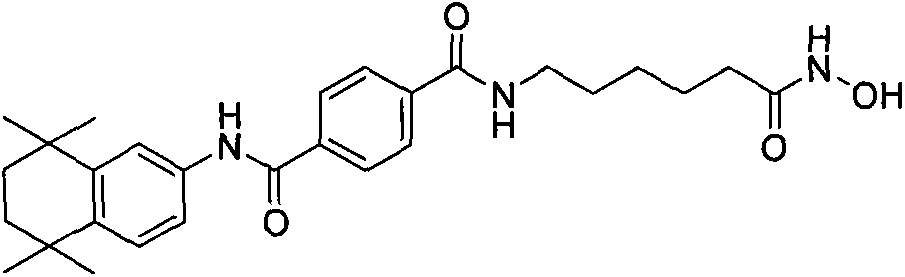
[0011] 1. Synthesis process: as shown in Figure 1.
[0012] 2. Experimental operation
[0013] (1) Synthesis of 2,5-dichloro-2,5-dimethylhexane
[0014] 2,5-Dimethyl-2,5-hexanediol (7.31g, 50mmol) was added to 12mol / L hydrochloric acid (60ml), heated to 85°C and stirred for 1h, cooled to room temperature, filtered with suction, and the filter cake was dissolved in Dichloromethane, washed with water until neutral, dried over anhydrous magnesium sulfate, filtered, the filtrate was evaporated to remove dichloromethane, and the residue was dried at room temperature under reduced pressure (0.7-13.3Pa) to obtain a white powdery solid 2,5-bis Chloro-2,5-dimethylhexane (7.50 g, 81.88%).
[0015] (2) 1,2,3,4-tetrahydro-1,1,4,4-tetramethylnaphthalene
[0016] Add 2,5-dichloro-2,5-dimethylhexane (7.32g, 40mmol) to benzene (80ml, 900mmol), add anhydrous aluminum chloride (0.53g, 4mmol) under stirring, and heat The reaction solution was refluxed for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com