Use of albiflorin in preparation of laryngeal cancer resistant medicines
A technology of paeonifloride glycosides and uses, applied in the field of paeonifloride glycosides in the preparation of anti-tumor drugs, which can solve the problems of limited quality control, difficulty in mass screening of anti-tumor active ingredients, and complex composition of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 The effect of paeoniflorin on tumor cell proliferation
[0031] Experimental scheme: Thiazolyl blue (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide, MTT) experiment was used, and paeoniflorin was used to treat rhabdomyosarcoma cells A204, Nasopharyngeal cancer cells CNE-2Z, intestinal cancer cells CT26, intestinal cancer cells HT29, neuroblastoma cells SY5Y, laryngeal cancer cells Hep2, pancreatic cancer cells PANC1, cervical cancer cells Hela, breast cancer cells MDA231, breast cancer cells T47D, Breast cancer cells MCF7, skin squamous cell carcinoma cells A431, kidney cancer Wilms cells G401, and prostate cancer cells DU145 for about 24 hours; after MTT staining for 4 hours, dimethyl sulfoxide (DMSO) was dissolved, and the absorbance value was measured at 570 nm by a microplate reader .
[0032] Experimental results: Paeoniflorin was found to be effective in pancreatic cancer cells PANC1, intestinal cancer cells CT26, intestinal cancer cells HT2...
Embodiment 2
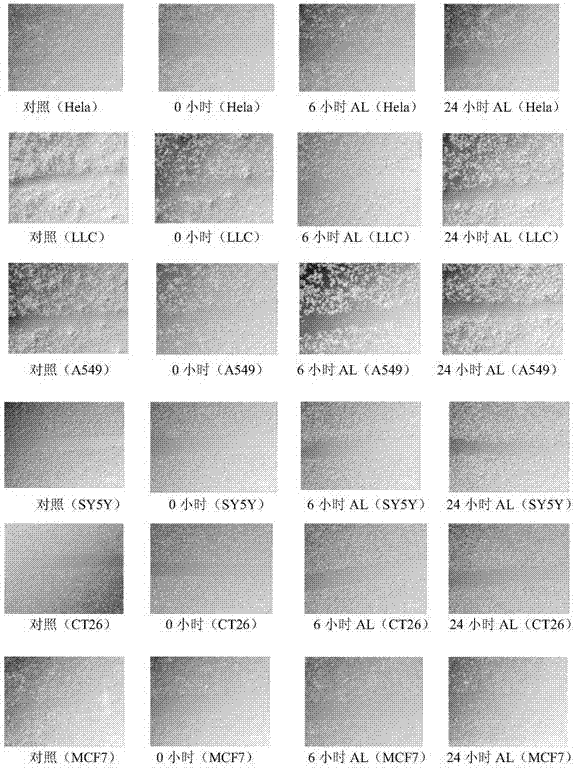
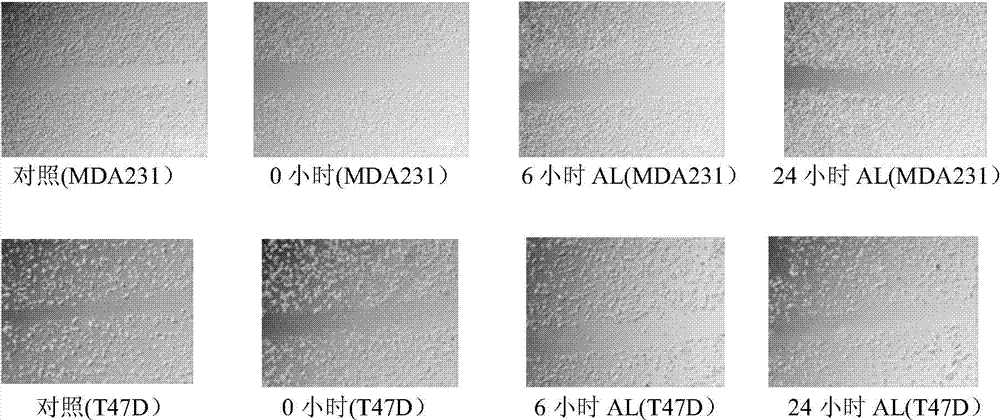
[0036] Example 2 The effect of paeoniflorin on tumor cell migration (Transwell assay and Scratch Analysis assay)
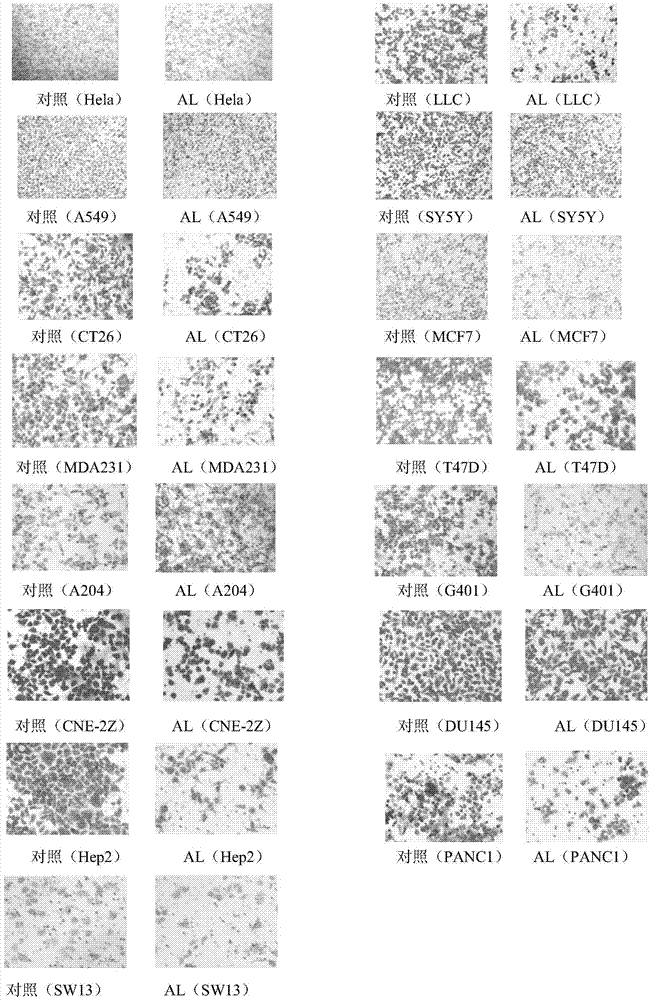
[0037] Experimental protocol: 1) Using cell migration assay (Transwell) assay, A549, LLC, Hela, SY5Y, CT26, T47D, MCF7, MDA231, A204, G401, CNE-2Z, DU145, Hep2, PANC1 and SW13 tumor cells were dosed After 24 hours of paeoniflorin (a safe dose of 20 μg / ml was selected), the cells were fixed in anhydrous ice methanol for 20 minutes, stained with crystal violet for 15 minutes, and photographed under a 100-fold light microscope to detect the number of cells migrated.
[0038] 2) Using cell migration assay (Scratch Analysis), to Hela, LLC, A549, SY5Y, CT26, MCF7, MDA231 and T47D cells plus paeoniflorin for 6 hours and 24 hours (select a safe dose of 20μg / ml), 40 times Light microscope photographs were taken to detect cell spacing.
[0039] Experimental results: 1) Transwell experiments showed that paeoniflorin (AL) can significantly inhibit lung cancer cells A549, lun...
Embodiment 3
[0042] Example 3 Comparison of the effect of paeoniflorin and total glucosides in paeony on tumor inhibition
[0043] 1. Comparison of the effects of paeoniflorin and total glucosides of paeony on tumor cell proliferation (MTT)
[0044] Experimental scheme: Thiazolyl blue (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide, MTT) experiment was used, and paeoniflorin and total glucosides of paeony were treated respectively A204, CNE-2Z, CT26, Hep2 and PANC1 tumor cells were stained for 24 hours, then MTT was stained for 4 hours, dimethyl sulfoxide (DMSO) was dissolved, and the absorbance value was measured at 570 nm by a microplate reader.
[0045] Experimental results: It was found that paeoniflorin and total glucosides of paeony have inhibitory effects on the proliferation of rhabdomyosarcoma cells A204, nasopharyngeal cancer cells CNE-2Z, intestinal cancer cells CT26, laryngeal cancer cells Hep2 and pancreatic cancer cells PANC1. IC of lactones on these tumors 50 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com