Continuous pegylation reaction method for recombinant human erythropoietin (EPO)
A technology of PEGylation and erythropoietin is applied in the field of chemical modification of proteins, which can solve the problems of increasing the difficulty of purification process and difficult to achieve specific site-specific PEGylation synthesis.
Active Publication Date: 2013-08-21
SHENZHEN SCIPROGEN BIO PHARMA
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, the relatively successful PEG-EPO drug in the world is "Mircera" listed by Roche. The single substitution reaction rate of its synthesis process is about 40%, and the content of multi-substituted products reaches 38%. The single-substituted product is PEG. -The target product of the EPO synthesis reaction has a relatively definite molecular structure and high drug activity, while the multi-substituted product is a by-product of the reaction, which will increase the difficulty of the subsequent purification process
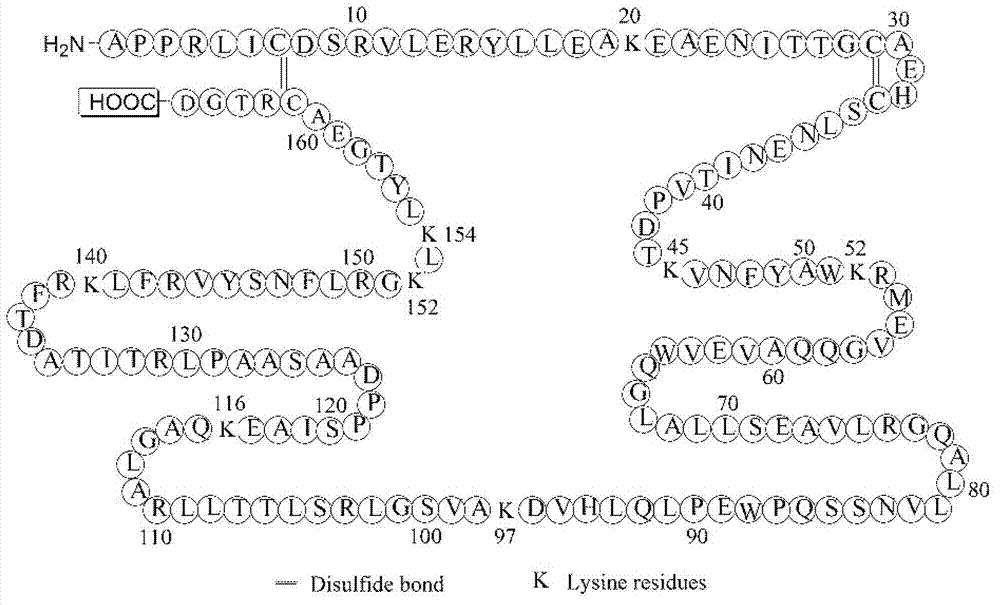
In addition, compared with other existing PEGylated protein drugs, EPO has 9 reaction sites (such as figure 1 shown), including 8 lysine residues and an N-terminal amino group, it is easy to form multiple substitution products in the reaction, and it is more difficult to achieve specific site-directed PEGylation
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
specific Embodiment
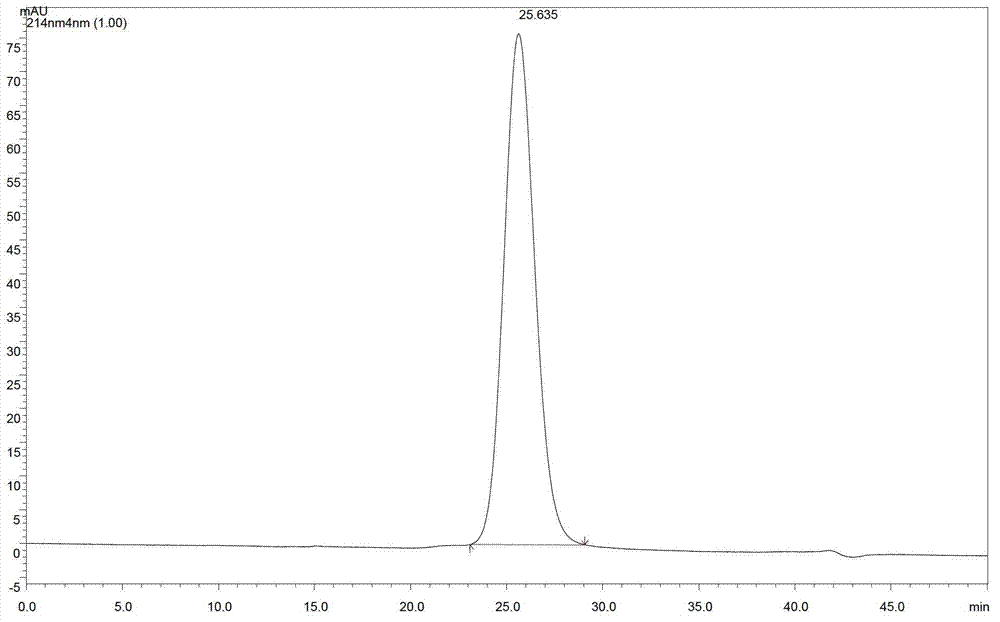
[0069] Specific embodiment: three consecutive PEGylation reaction methods prepare M-PEG-EPO reaction solution
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Login to View More
Abstract
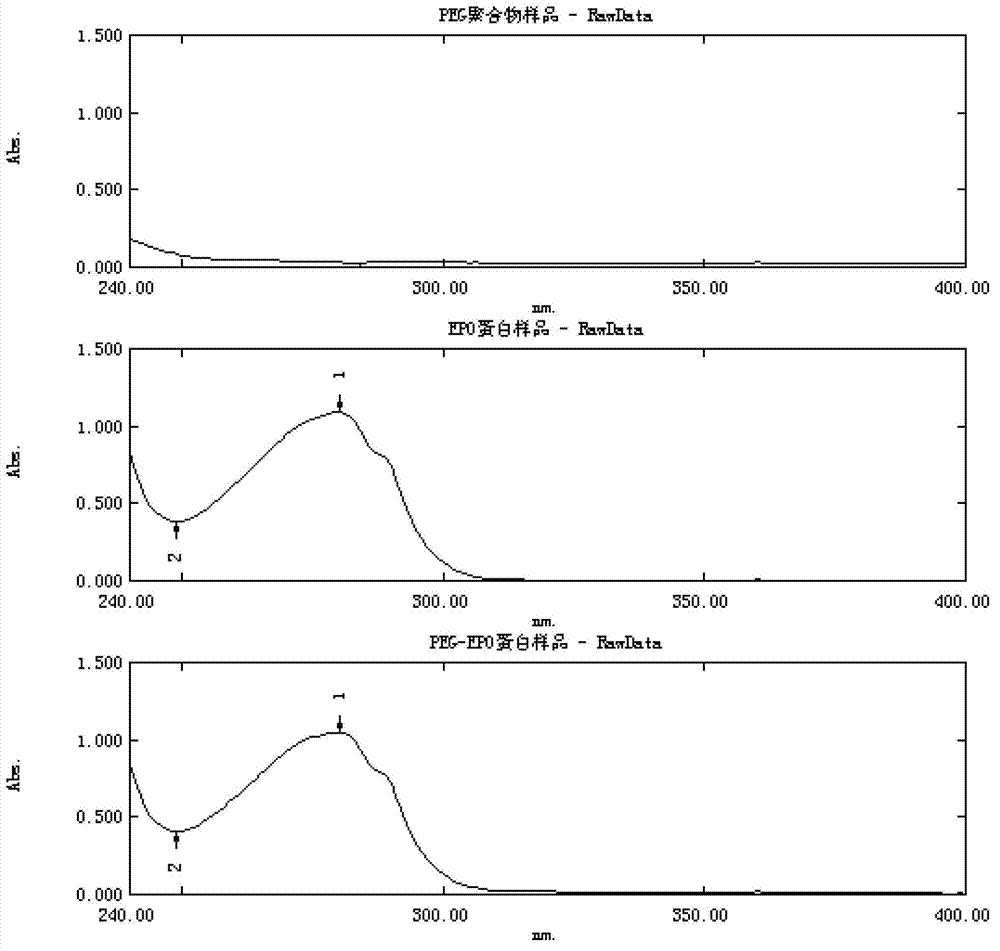
The invention relates to chemical modification of proteins, and discloses a continuous pegylation reaction method for recombinant human erythropoietin (EPO). The method comprises the following steps of: performing a primary pegylation reaction on EPO molecules in a first buffer solution; and further adding PEG into a reaction liquid obtained in the primary pegylation reaction in a second buffer solution which is different from the first buffer solution for performing a secondary pegylation reaction to obtain a final reaction liquid. A reaction method of multiple buffering systems is applied, so that continuous pegylation reaction of EPO is realized, and a pegylation reaction method with high mono-substitution rate and low multi-substitution rate is established.
Description
technical field [0001] The present invention relates to protein chemical modification, in particular to a continuous pegylation reaction method of recombinant human erythropoietin. Background technique [0002] Recombinant human erythropoietin (EPO for short), is a specific hematopoietic factor that protects and promotes the differentiation, proliferation and maturation of hematopoietic stem cells in the bone marrow into red blood cells. Renal anemia and non-renal anemia are known as genetic engineering drugs with the most mature technology, the most definite curative effect and the highest global sales. However, the drug has major problems such as frequent medication (one or three injections per week) and low biological activity, which brings great treatment pain and inconvenience to patients. Therefore, there is a need for further modification and improvement, that is, the development of long-acting EPO drugs. At present, one of the more mature long-acting EPO drugs in t...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07K17/08
Inventor 陈贤光盛光阳张涤平吴园园张向荣黎雄辉郑崇吉
Owner SHENZHEN SCIPROGEN BIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com