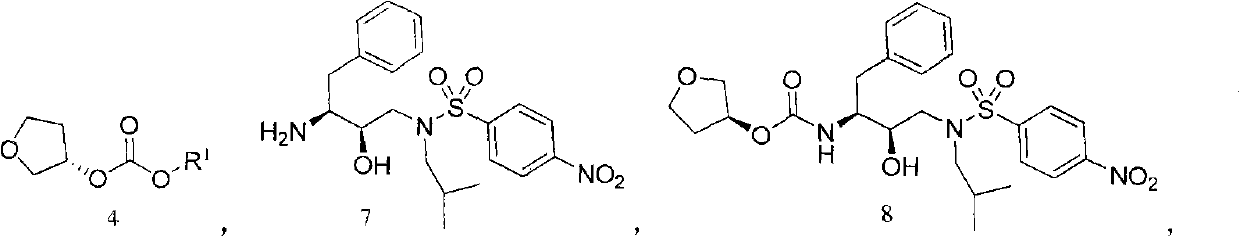
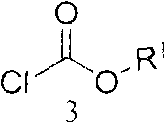
Method for preparing fosamprenavir intermediate
A technology of fusarnavir and intermediates, applied in the field of heterocyclic chemistry, can solve the problems of low activity of tetrahydrofuran-3-chloroformate, low yield of active esters, expensive active reagents, etc., and achieve simple reaction types , easy operation, high industrial application and economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: the preparation of formula 4-1 compound
[0059]
[0060] (S)-tetrahydrofuran-3-ol (3.52g, 0.04mol) and triethylamine (5.05g, 0.05mol) were mixed and dissolved in 30ml of dichloromethane, and methyl chloroformate (4.25g, 0.045mol) was added dropwise to In the reactor, after the dropwise addition is completed, the reaction is continued at 30-50° C. for 3-10 hours. TLC detects that the raw material disappears, and a quantitative crude compound of formula 4-1 is obtained.
Embodiment 2
[0061] Embodiment 2: the preparation of formula 4-2 compound
[0062]
[0063] According to the method of Example 1, phenyl chloroformate (6.24g, 0.04mol) and sodium carbonate (8.48g, 0.08mol) were operated in the same way to obtain a quantitative compound of formula 4-2.
Embodiment 3
[0064] Embodiment 3: the preparation of formula 4-3 compound
[0065]
[0066] According to the method of Example 1, benzyl chloroformate (6.8 g, 0.04 mol) and pyridine (4.74 g, 0.06 mol) were operated in the same way to obtain a quantitative compound of formula 4-2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com