Antibodies that specifically bind to A beta oligomers and use thereof
A technology of oligomers and antibodies, applied in the direction of antibodies, specific peptides, drug combinations, etc., can solve the problems of difficult to measure Aβ oligomers alone, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[4596] Hereinafter, the present invention is specifically described with reference to examples, but should not be construed as being limited thereto.
[4597] method :
[4598] Antigen preparation
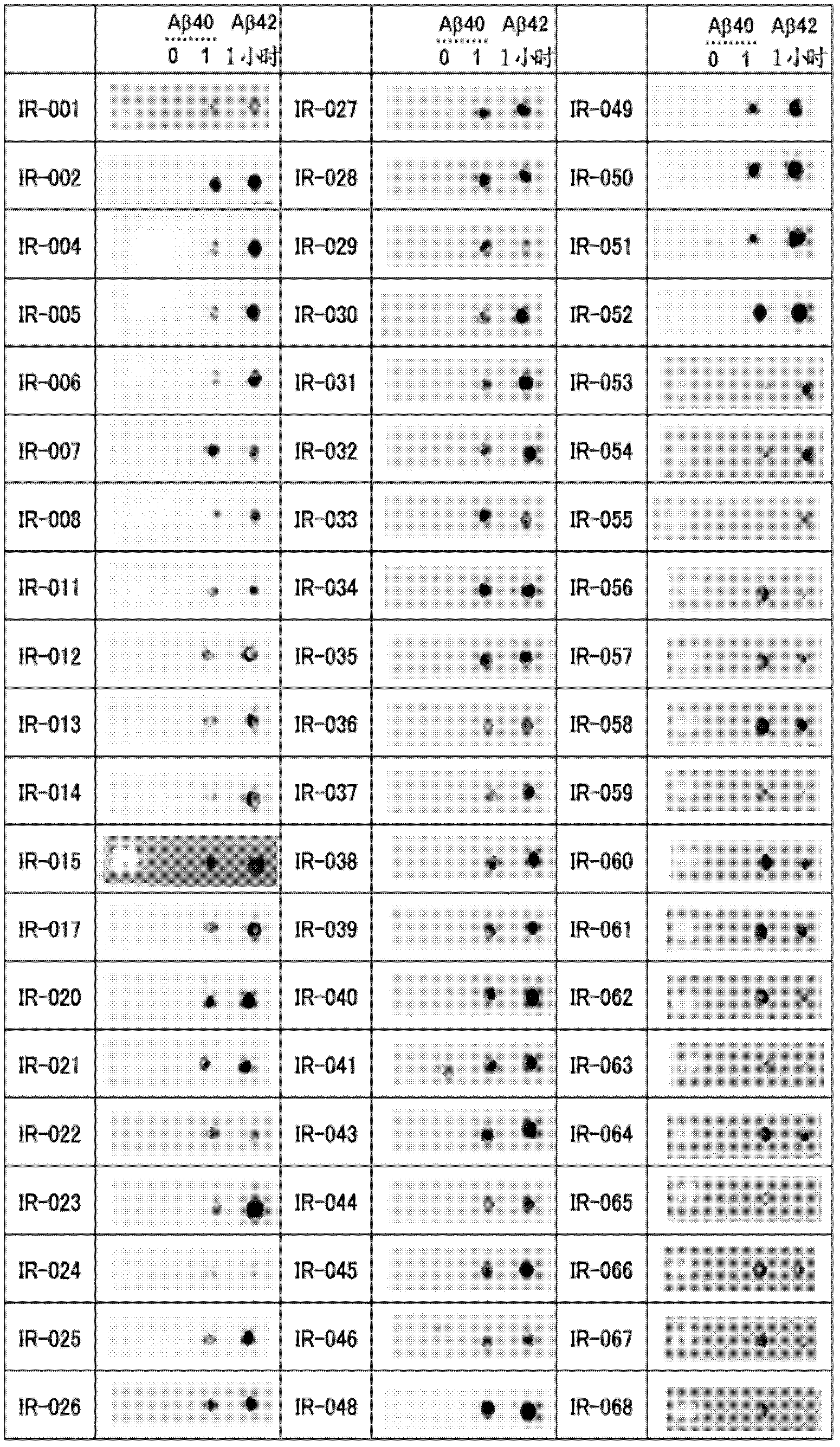
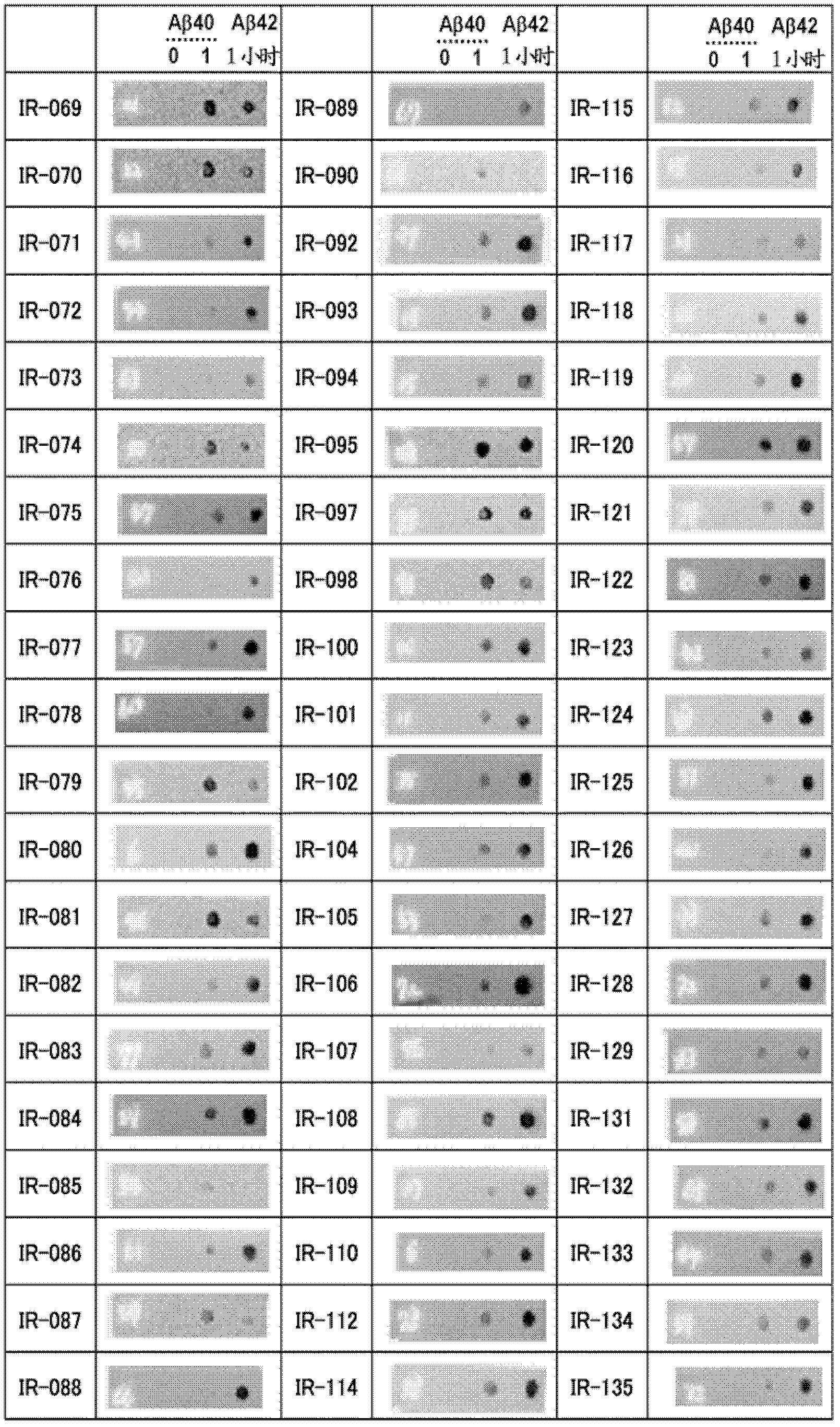
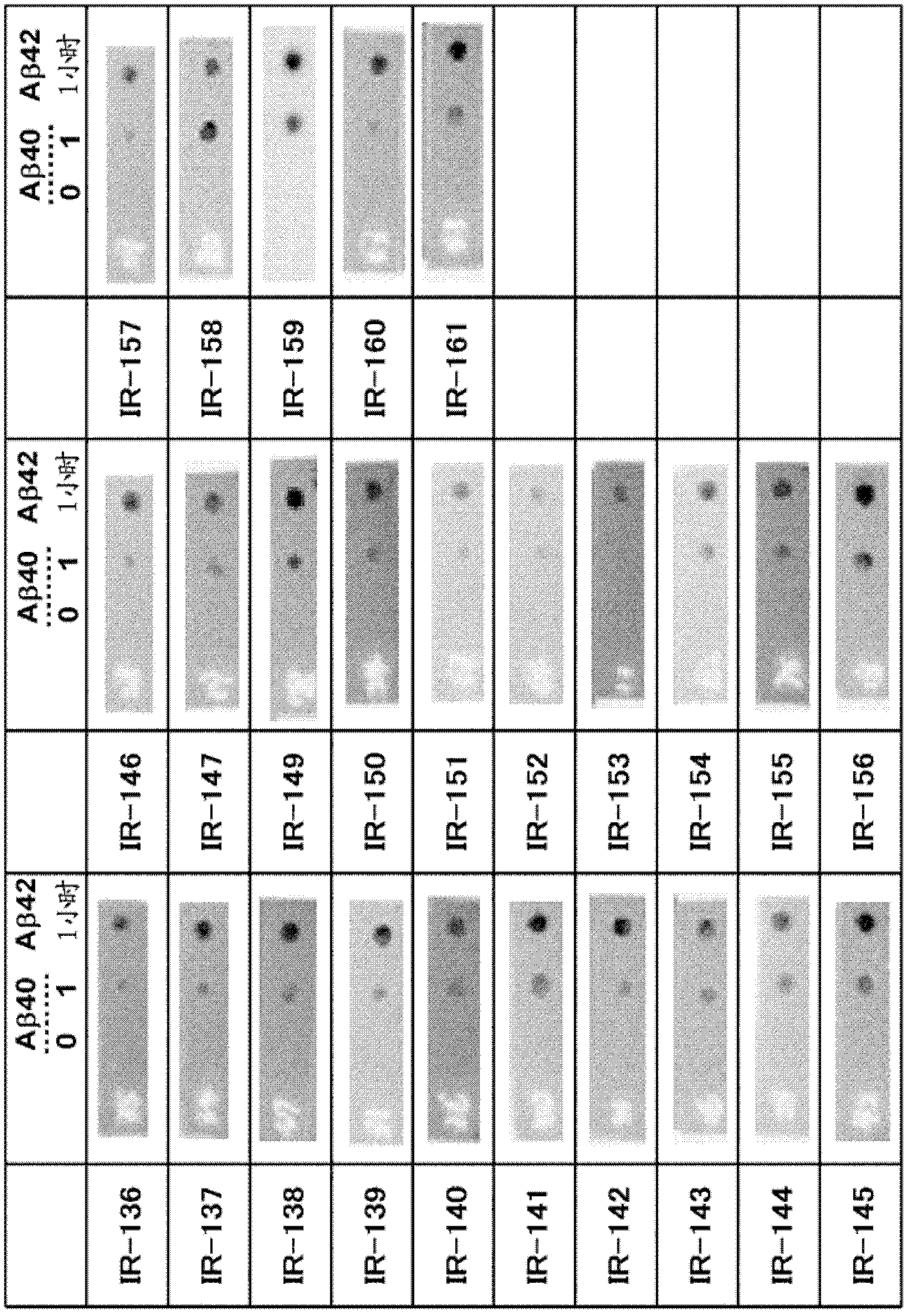
[4599] The fluorescent dye 6-carboxytetramethylrhodamine (6-TAMRA) (SIGMA) was chemically linked to the N-terminus of the synthetic Aβ1-40 peptide (Peptide Institute, Inc.) to generate modified Aβ. An oligomer-rich sample (Aβ1-40 oligomer) was prepared by copolymerizing the modified Aβ and the synthetic Aβ1-40 peptide. Conditions are preferably adjusted so that the fluorescence intensity measured by the ThT assay described below (Yamamoto N, et al: J Biol Chem, 282: 2646-2655, 2007) is a quarter or less of that in the absence of modified Aβ . More specifically, it is preferable to mix 100 µM each of the modified Aβ and the synthetic Aβ 1-40 peptide, and polymerize for 20 hours.
[4600] Preparation of antibody-producing hybridomas
[4601] BALB / c mice were immunized by i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com