Dry suspension of cefpodoxime proxetil composition and preparation method thereof
A technology of cefpodoxime axetil and dry suspension, applied in the field of medicine, can solve problems such as unfavorable swallowing, poor patient taking compliance, poor dissolution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
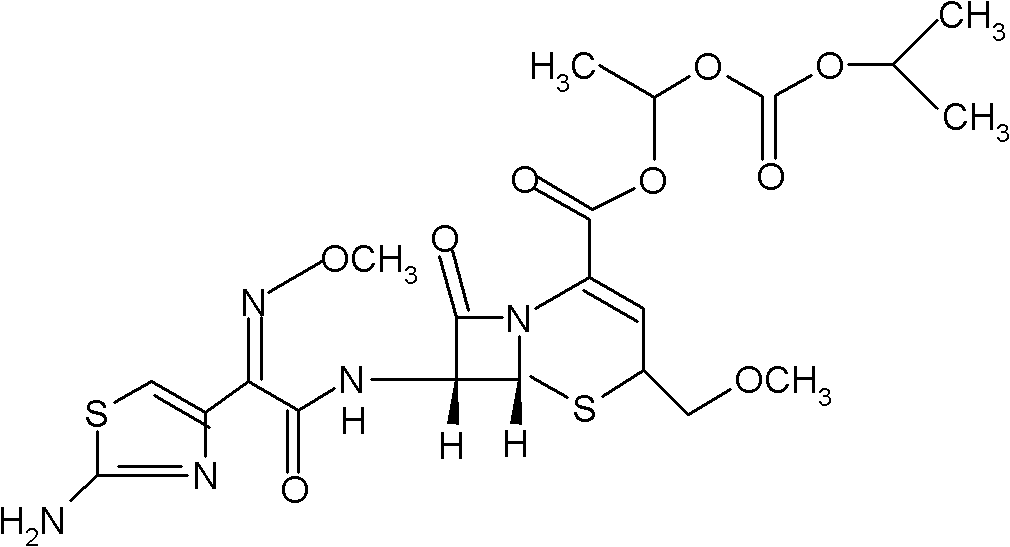
[0018] Embodiment 1: dry suspension of cefpodoxime axetil composition of the present invention
[0019]
[0020] Preparation method: take cefpodoxime axetil and sucrose to pass through a 100-mesh sieve respectively, and take hypromellose and xanthan gum to pass through a 120-mesh sieve respectively; take 50 g of pulverized cefpodoxime axetil and 660 g of pulverized sucrose, mix evenly, add 200g of 95% ethanol is used to make soft materials, and then granulated with a 40-mesh sieve. The prepared wet granules are dried at 45-50°C for 30 minutes, and granulated through a 40-mesh sieve; Mix with pulverized xanthan gum 71g to obtain.
Embodiment 2
[0021] Embodiment 2: the antibacterial effect of cefpodoxime axetil composition dry suspension of the present invention
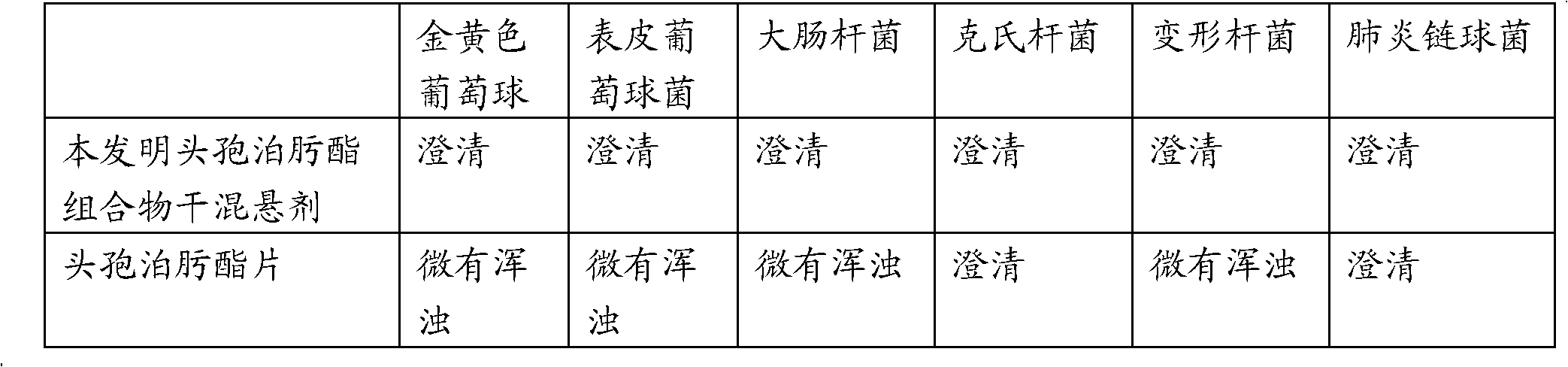
[0022] Inoculate one piece of Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella, Proteus mirabilis, and Streptococcus pneumoniae in 1 mL of broth medium, and culture them at 37°C for 8-9 hours to make the meat of the bacteria. soup suspension. Get 12 sterile test tubes, every six as a batch, divide into two batches, add the broth suspension of above-mentioned six kinds of bacteria respectively in each batch, then add 6 parts of 1mL containing 0.5mg cefpodoxime axetil composition of the present invention The dry suspension broth culture medium was added to a batch of broth suspensions of six kinds of bacteria respectively, and the broth culture medium containing 0.5 mg of the existing cefpodoxime axetil tablet was treated in the same way as above, and then cultivated at 37 ° C for 24 hours, and observed The results are shown in...
Embodiment 3
[0026] Example 3: Clinical research on the antibacterial effect of the dry suspension of the cefpodoxime axetil composition of the present invention
[0027] 2000 patients with upper respiratory tract infection, lower respiratory tract infection, skin and soft tissue infection, and genitourinary system caused by sensitive bacteria were randomly divided into treatment group A, treatment group B and control group. Treatment group A is given existing cefpodoxime axetil tablet on the basis of routine treatment, and treatment group B is given the dry suspension of cefpodoxime axetil composition prepared by the embodiment of the present invention 1 on the basis of routine treatment, every day 2 times, 100 mg each time, 7 days of treatment, the control group was given cephalexin. The bacterial clearance rate of each group was counted, and the results are shown in Table 2
[0028] Table 2 Antibacterial effect clinical research
[0029] control group
[0030] Sig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com