Method for preparing penehyclidine hydrochloride injection
A technology of penehyclidine hydrochloride and injection, which is applied in the field of medical drugs, can solve problems such as poor stability, decreased content, and increased pH value, and achieve the effects of easy product quality, stable content, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
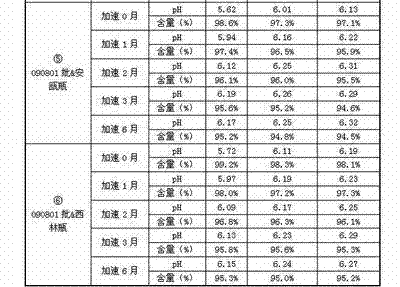
Embodiment 1
[0152] Example 1 (adjustment of pH & ampoule)
[0153] prescription:
[0154] Penehyclidine Hydrochloride 20g
[0155] Add water for injection to 20000ml
[0156] (Specification: 1ml: 1mg, 1ml per tube)
[0157] Preparation Process:
[0158] Weigh 20.0g of penehyclidine hydrochloride, add 16000ml of water for injection to dissolve, adjust pH=4.72 with 0.1mol / L hydrochloric acid solution, add water for injection to the full amount, measure pH=4.88, content=101.5%. The medicinal solution is filtered through a 0.22 μm filter membrane and filled into washed and dried ampoules. The samples obtained by potting were sterilized at 121° C. for 12 minutes. After passing the inspection, the sample of Example 1 is obtained.
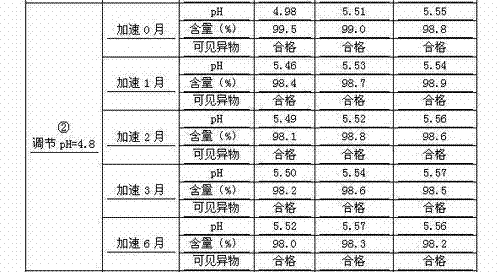
Embodiment 2
[0159] Embodiment 2 (regulate pH & vial)
[0160] prescription:
[0161]Penehyclidine Hydrochloride 10g
[0162] Add water for injection to 20000ml
[0163] (Specification: 1ml: 0.5mg, 1ml per tube)
[0164] Preparation Process:
[0165] Weigh 10.0g of penehyclidine hydrochloride, add 16000ml of water for injection to dissolve, adjust pH=4.75 with 0.5mol / L hydrochloric acid aqueous solution, add water for injection to the full amount, measure pH=4.87, content=100.7%. The medicinal solution was filtered through a 0.22 μm filter membrane and then sealed in a cleaned and dried vial. The samples obtained by potting were sterilized at 121° C. for 12 minutes. The sample of Example 2 is obtained after passing the inspection.
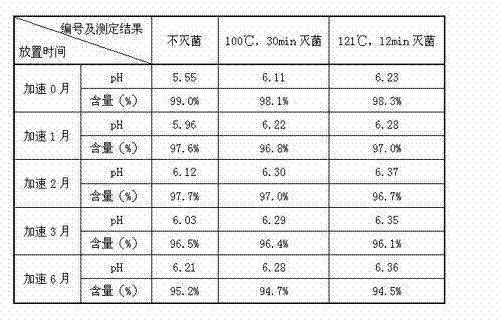
Embodiment 3
[0166] Embodiment 3 (regulate pH & vial)
[0167] prescription:
[0168] Penehyclidine Hydrochloride 10g
[0169] Add water for injection to 20000ml
[0170] (Specification: 1ml: 0.5mg, 1ml per bottle)
[0171] Preparation Process:
[0172] Weigh 10.0g of penehyclidine hydrochloride, add 16000ml of water for injection to dissolve, adjust pH=4.65 with 0.3mol / L hydrochloric acid aqueous solution, add water for injection to the full amount, measure pH=4.87, content=100.7%. The medicinal solution was filtered through a 0.22 μm filter membrane and then sealed in a cleaned and dried vial. The samples obtained by potting were sterilized at 121° C. for 12 minutes. The sample of Example 2 is obtained after passing the inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com