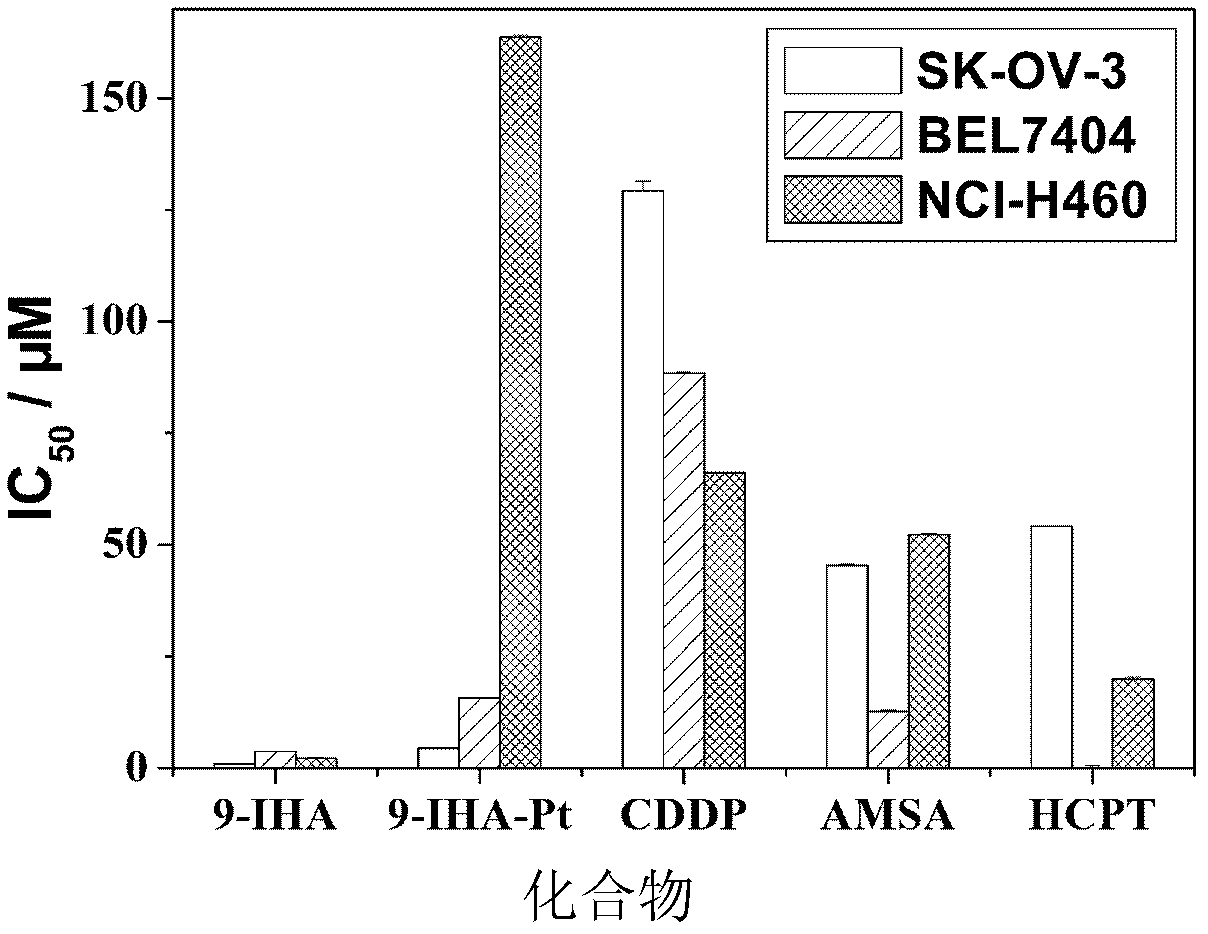
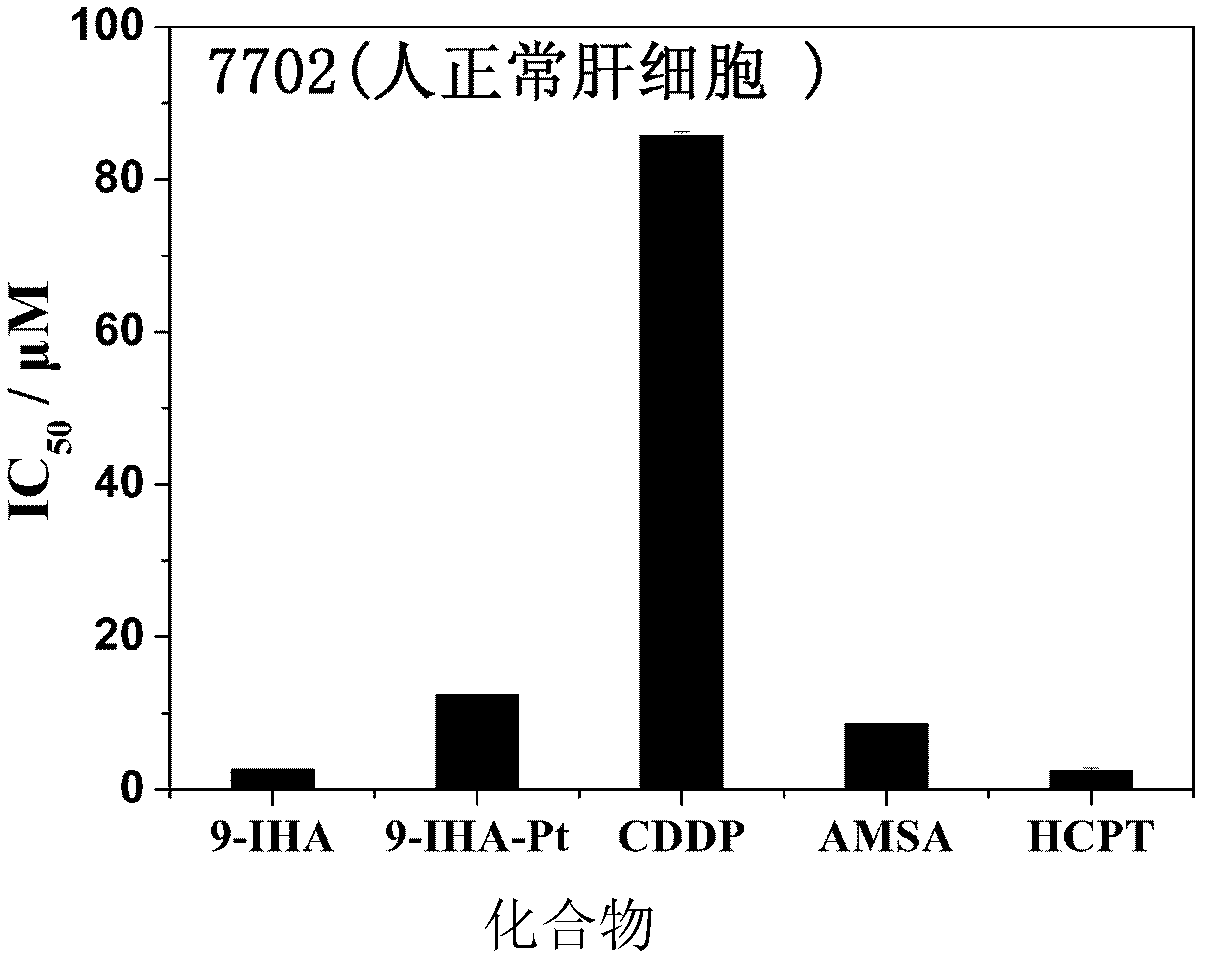
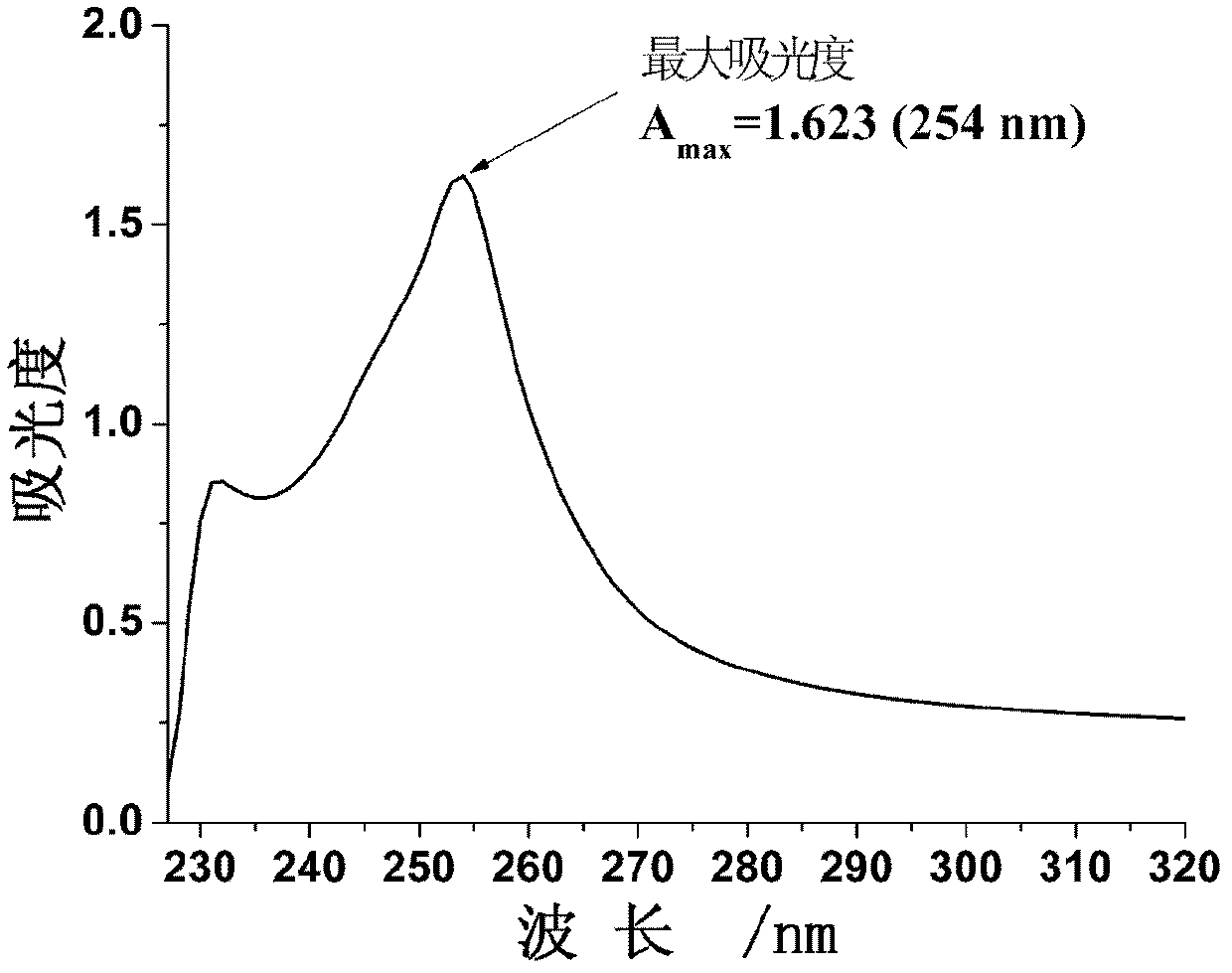
9-anthracenecarboxaldehyde-4,5-dihydro-1h-imidazol-2-yl-hydrazone cisplatin complex and synthesis method and use thereof
A technology of complexes and synthesis methods, applied in the fields of compounds containing elements of Group 8/9/10/18 of the periodic table, chemical instruments and methods, organic chemistry, etc. Problems such as drug resistance, single target and mechanism of action, etc., to achieve the effect of high medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: direct synthesis of cis-[Pt by normal pressure solution method II Cl 2 (9-IHA)]
[0031] Will K 2 PtCl 4 (1.0mmol, 0.415g) and 9-IHA (1.0mmol, 0.290g) were dissolved in 5mL freshly distilled anaerobic water and 50mL methanol, and then dissolved K 2 PtCl 4 The aqueous solution of 9-IHA was added dropwise to the methanol solution of 9-IHA, and the reaction was carried out at 30°C for 48 hours under the protection of nitrogen in the dark, and a large amount of dark brown precipitate was formed, which was the target product. Cool and filter, wash with water, methanol, and diethyl ether several times in small amounts, place in a vacuum desiccator, and dry at 30°C until constant weight.
Embodiment 2
[0032] Embodiment 2: normal pressure solution method synthesis cis-[Pt II Cl 2 (9-IHA)]
[0033] Na 2 PtCl 4 (1.0mmol, 0.400g) and 9-IHA (1.0mmol, 0.290g) were dissolved in 20mL H 2 O and 100mL ethanol, the ethanol solution dissolved with 9-IHA was gradually added dropwise to Na 2 PtCl 4 In the aqueous solution, under the protection of light and nitrogen, reflux at 80°C for 2 hours, and evaporate most of the solvent. After cooling, a large amount of dark brown precipitates are formed, which is the target product. Cool and filter, wash with water, ethanol, and acetone several times successively, place in a blast dryer, and dry at 60°C until constant weight.
Embodiment 3
[0034] Embodiment 3: Atmospheric solution method synthesizes cis-[Pt by intermediate II Cl 2 (9-IHA)]
[0035] The Pt(II) intermediate cis-[PtCl 2 (DMSO) 2 ] (1.0mmol, 0.422g) was heated and dissolved in 30mL of methanol, 9-IHA (1.0mmol, 0.290g) was dissolved in 50mL of methanol, and then the methanol solution of the Pt(II) intermediate was gradually added dropwise to 9- In the methanol solution of IHA, reflux for 1 hour at 65°C in the dark and under the protection of nitrogen. After evaporating most of the methanol, cool to room temperature. A large amount of dark brown precipitates are formed, which is the target product. Cool and filter, wash with water, methanol and diethyl ether several times successively, place in a vacuum desiccator, and dry at 40°C until constant weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com