New therapy and medicament using integrin ligands for treating cancer
A technology of integrin and ligand, which is applied in the direction of drug combination, peptide/protein composition, medical preparations containing active ingredients, etc., can solve the problem of reducing curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0589] The following flow chart describes an example of a method of treatment comprising application of whole brain radiation and administration of cyclo-(Arg-Gly-Asp-DPhe-NMeVal) and / or a pharmaceutically acceptable salt thereof, preferably cyclo-(Arg-Gly- Asp-DPhe-NMeVal) (= Cilengitide):
[0590]
[0591] The following flow chart describes an example of a study design for a therapeutic approach consisting of application of whole brain radiation and administration of cyclic-(Arg-Gly-Asp-DPhe-NMeVal) and / or a pharmaceutically acceptable salt thereof, preferably cyclic-(Arg -Gly-Asp-DPhe-NMeVal) (= Cilengitide) composition:
[0592]
[0593] Particularly preferred subjects of the invention are all uses, methods of treatment, medicaments, individuals of the invention, embodiments of the invention and / or methods of preparation described herein
[0594] i) It comprises the administration of at least one specific integrin ligand in one or more doses such that the weekly dos...
Embodiment 1
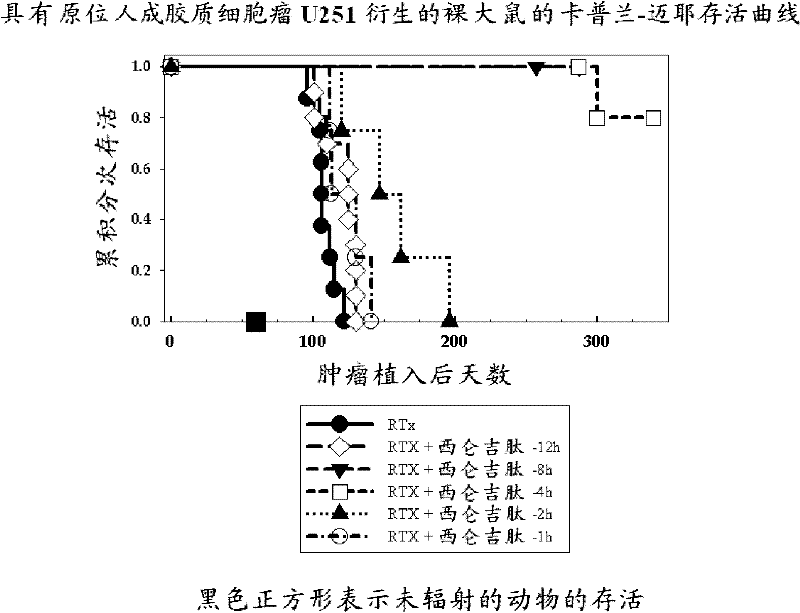
[1138] Example 1: Radiotherapy, Cilengitide (= Cyclo-(Arg-Gly-Asp-DPhe-Nme-Val)) Timing Experiment in a Rat Orthotopic Glioblastoma Model
[1139] NIH rnu nude rats were anesthetized, bound, and injected intracerebrally with 5×10E5U251 human glioblastoma cells suspended in 10 μL of cell culture medium at a depth of 1 mm behind the orbit, 3 mm and 2.5 mm to the right of the anterior blemish, A #2701 Hamilton syringe fitted with a 26-gauge needle was used, and injections were performed essentially as previously described (Engebraaten et al., 1999). 14 days later, at various times prior to monotherapy with application of a single, parallel, dorsal-ventral 6MV x-ray beam so that 95-100% of the central axis dose of 25 Gy hits the tumor volume (Kim et al., 1999) (8h, 4h, 2h, 1h), Cilengitide (4 mg / kg) in PBS was administered as an intraperitoneal bolus. Animals also received the same intraperitoneal bolus of cilengitide each day for the next 7 days. Animals were maintained under c...
Embodiment 2
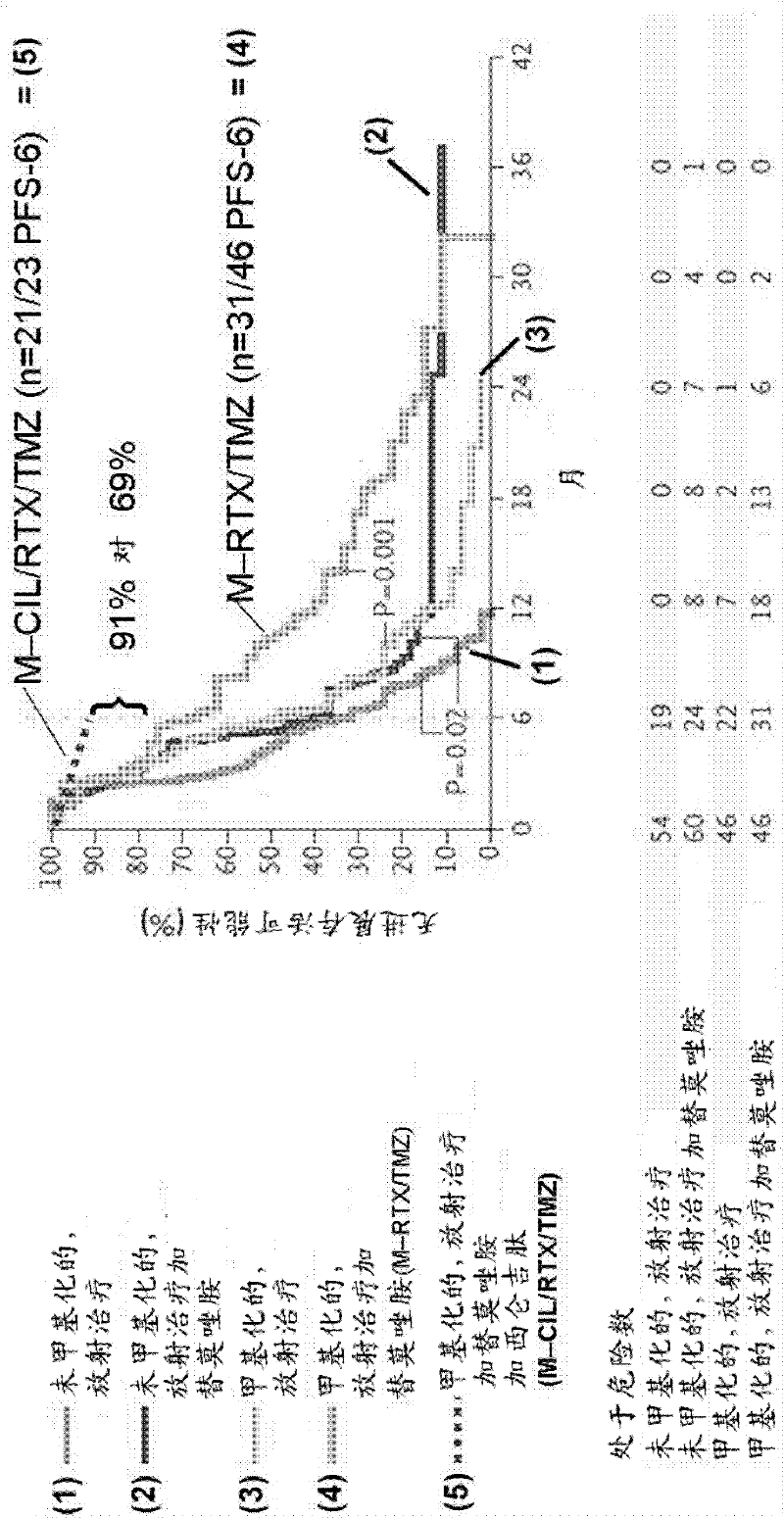
[1153] Example 2: Phase IIa trial of cilengitide ((=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val)) single agent therapy in patients with recurrent glioblastoma
[1154] Background: This phase IIa study was designed to evaluate the cyclic RGD pentapeptide cilengitide ((=cyclo-(Arg-Gly-Asp-DPhe-NMe-Val), an inhibitor of integrins avβ3 and avβ5) as a single active agent , safety, toxicity and clinical activity in patients (pts) with relapsed glioblastoma (GBM) at doses of 500 mg and 2000 mg.
[1155]METHODS: In this multicentre, open-label, randomized, and uncontrolled study, pts with recurrent GBM and detectable disease following previous treatment with temozolomide and radiation therapy were randomized to receive a dose of 500 mg Or 2000mg of cilengitide intravenously twice a week until disease progression. Independent blinded examinations were performed for histopathological diagnosis and MRI imaging. The primary endpoint was progression-free survival (PFS) at 6 months (mths). Secondary...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com