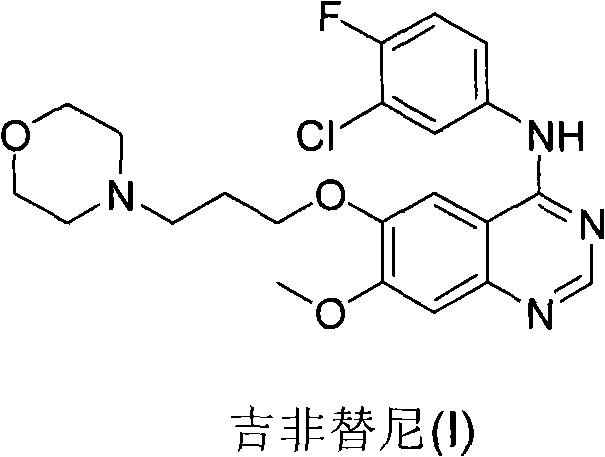
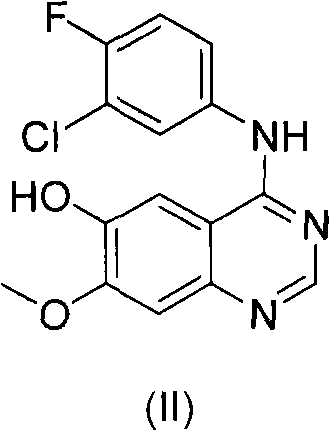
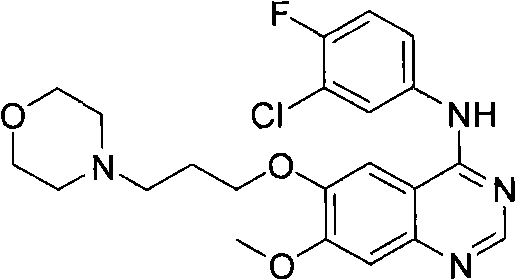
Preparation method of Gefitinib
A technology of gefitinib and triphenylphosphine, applied in the field of chemical drug preparation, can solve problems such as the yield of only 50%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] As far as the preparation method of the present invention is concerned, the reactant formula (II) compound and 4-(3-hydroxypropyl)-morpholine (i.e. 3-morpholinopropanol) are commercially available, or can be Preparation by methods known per se to the skilled person.
[0033] In a preferred embodiment of the method of the present invention, the molar ratio of 4-(3-hydroxypropyl)-morpholine to the compound of formula (II) is 1:1-10:1.
[0034] In a preferred embodiment of the method of the present invention, diisopropyl azodicarboxylate and triphenylphosphine are used as reaction accelerators.
[0035] In a preferred embodiment of the method of the present invention, the molar ratio of triphenylphosphine to diisopropyl azodicarboxylate is 1:1-10:1 or 1:1-1:10.
[0036] In a preferred embodiment of the method of the present invention, the molar ratio of triphenylphosphine to the compound of formula (II) is 1:1-10:1.
[0037] In the reaction of the method of the present i...
Embodiment
[0043] Embodiment: the preparation of gefitinib
[0044]
[0045] To a solution of triphenylphosphine (2.01 g, 7.66 mmol) in THF (13 mL) was added diisopropyl azodicarboxylate (1.5 mL, 7.66 mmol) in THF (2 mL). The color of the clear solution turned to yellow. After 1 hour, a solution of 4-(3-hydroxypropyl)morpholine (0.23 mL, 1.69 mmol) in THF (2 mL) was added dropwise to the yellow solution at room temperature over 20 minutes. Then, under nitrogen, 4-(3'-chloro-4'-fluoroanilino)-6-hydroxy-7-methoxyquinazoline (0.50 g, 1.53 mmol) of formula (II) was added to the reaction in solution. The resulting mixture was stirred at room temperature for 4 hours, then a solution of 4-(3-hydroxypropyl)morpholine (0.19 mL, 1.38 mmol) in THF (2 mL) was added dropwise to the yellow reaction over 20 minutes. in solution. The resulting mixture was stirred for an additional 1 hour at room temperature. The reaction was terminated, and the solvent was removed by evaporation. The residue w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com