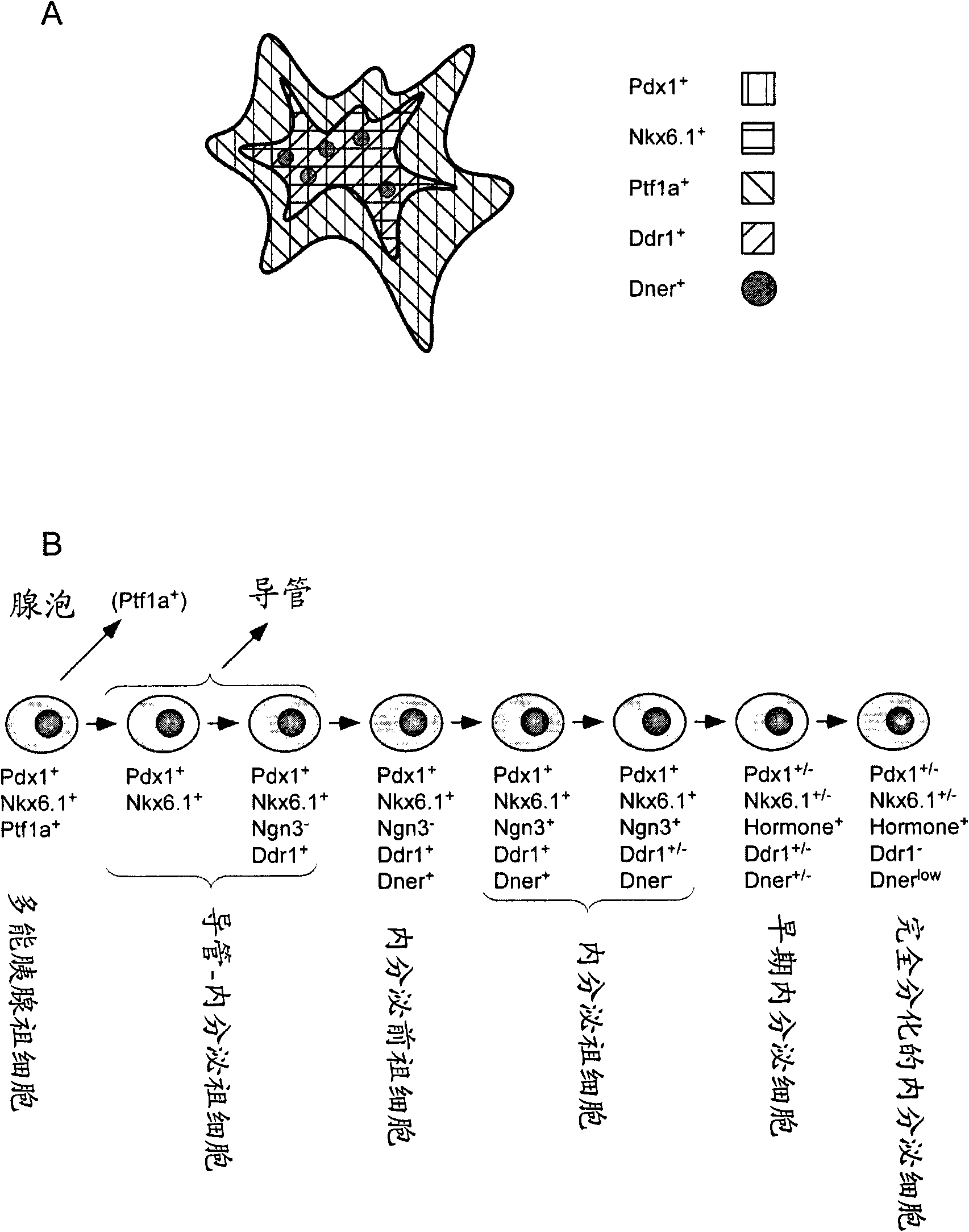
Dner-mediated cell purification of pancreatic endocrine pre-progenitor cells and their progeny
一种内分泌祖细胞、胰腺细胞的技术,应用在DNER介导的胰腺内分泌前祖细胞及其子代的细胞纯化领域,能够解决荧光增加等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Example 1: Bioinformatics Analysis of Mouse and Human Dner
[0228] To discover extracellular and transmembrane domains, mouse and human Dner were run through CBS's transmembrane predictor TMHMM server v.2.0 (http: / / www.cbs.dtu.dk / ). The results showed that both human and mouse Dner were predicted as single transmembrane proteins (type I), the main first 638 amino acids were on the extracellular side in both humans and mice, and the transmembrane domain was predicted to be amino acids 639-661. The transmembrane-like region predicted by TMHMM in the first part of the Dner protein is the signal peptide, which is also discussed below in the results related to SignalP.
[0229] Dner's mouse and human signal peptides were analyzed using CBS's SignalP 3.0 prediction server (http: / / www.cbs.dtu.dk / ). Mouse: signal peptide probability: 1.000; signal anchor probability: 0.000; maximum cleavage site probability: 0.494, between positions 39 and 40. Human: signal peptide probabili...
Embodiment 2
[0234] Example 2: Expression of Dner and Ddr1 in pancreatic beta cell lines
[0235]Total RNA was isolated from mouse endocrine-like cell lines: Ins1, βHC, βTCtet (condition: cultured with tetracycline (+tet)), βTCtet (condition: cultured without tetracycline (-tet)), Min6, αTC, and isolated from e15. 5 NMRI gastrointestinal tract (stomach, duodenum, spleen and pancreas) tissue. The cell line Ins1 is described in M Asfari et al., Endocrinology, Vol. 130, 167-178. The cell line BetaHC-9 is described in Noda M et al., Diabetes, 1996 Dec;45(12):1766-73. The cell line βTC-tet is described in Fleischer N et al., Diabetes, 1998 Sep;47(9):1419-25. The cell line Min6 is described in J Miyazaki et al., Endocrinology, 1990 Jul;127(1):126-32. The cell line αTC is described in Powers AC et al., Diabetes, 1990 Apr;39(4):406-14. Prepare randomly primed cDNA using RNA as template. These cDNAs were subjected to 35 rounds of PCR (96°C, 180 seconds, then (96°C, 30 seconds, 55°C, 30 secon...
Embodiment 3
[0237] Example 3: Expression of Dner and Ddr1 in the endocrine-like cell line αTC, visualized by IHC in chamber slides
[0238] αTC was grown in DMEM containing 1000 mg / L D-glucose, sodium pyruvate (0.5 mM), P / S (penicillin (10 IU / ml), streptomycin (10 μg / ml)) and 10% FCS. The cell line αTC is described in Powers AC et al., Diabetes, 1990 Apr;39(4):406-14. Cells were fixed with Lilliys fixative for 45 minutes, then washed with PBS and stored in PBS at +5°C until use. Infiltrate by ethanol gradient (start at 70%, change to 96%, then change to 99%, then change to 99%, then change to 96%, finally change to 70%, use 5 min incubation for each concentration) Cells were then blocked with 0.5% TNB blocking reagent (from Perkin-Elmer) and stained. Goat anti-Dner (R&D Systems, AF2254, 1:150) or mouse anti-Ddr1 (R&D Systems, MAB2396, 1:50) was then added and incubated overnight (O / N) at room temperature (RT). The next day, cells were washed with PBS and biotinylated anti-donkey or ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com