Method for preparing cellulose eleostearate
A technology of cellulose and eleostearate, which is applied in the field of homogeneous preparation of cellulose eleuate, can solve the problems of no continuous cross-linking reaction, inability to further form molecular weight products, etc., and achieves good thermoplastic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
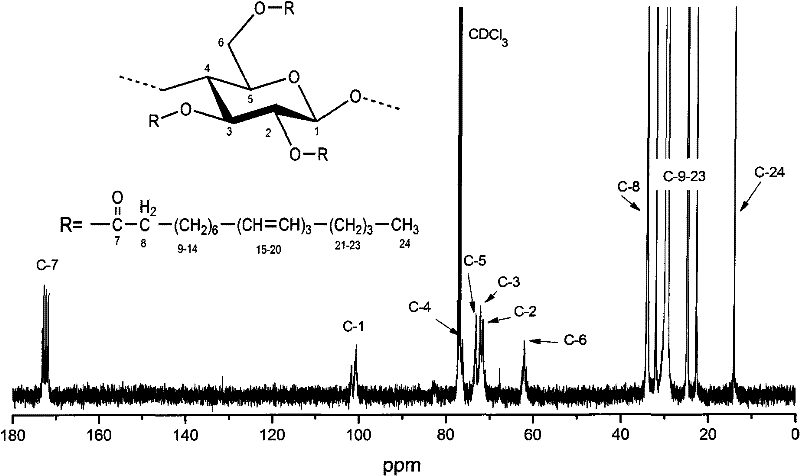
[0025] Add 1 gram of microcrystalline cellulose and 9 grams of 1-N-n-butyl-3-trimethylimidazolium chloride into a 50-ml round-bottomed flask, raise the temperature to 100°C, and stir for about 20 minutes to make the microcrystalline cellulose The element was completely dissolved in 1-N-n-butyl-3-trimethylimidazolium chloride. Heating was stopped, and the temperature of the solution was maintained at 90° C., 9.34 g of stearyl chloride was added dropwise, and the reaction was completed in 1.5 hours. Add 200 milliliters of methanol in the reaction flask, and stir for 10 minutes, the cellulose sulphonic acid ester is precipitated from the solution, and is suction-filtered under reduced pressure with a Buchner funnel, and then 400 milliliters of methanol are used to depressurize the cellulose sulphonic acid ester in Buchner's suction. Wash and suction filter in the filter funnel 3 times, store the filtrates after suction filtration, put the solid product on the filter paper into a ...
Embodiment 2
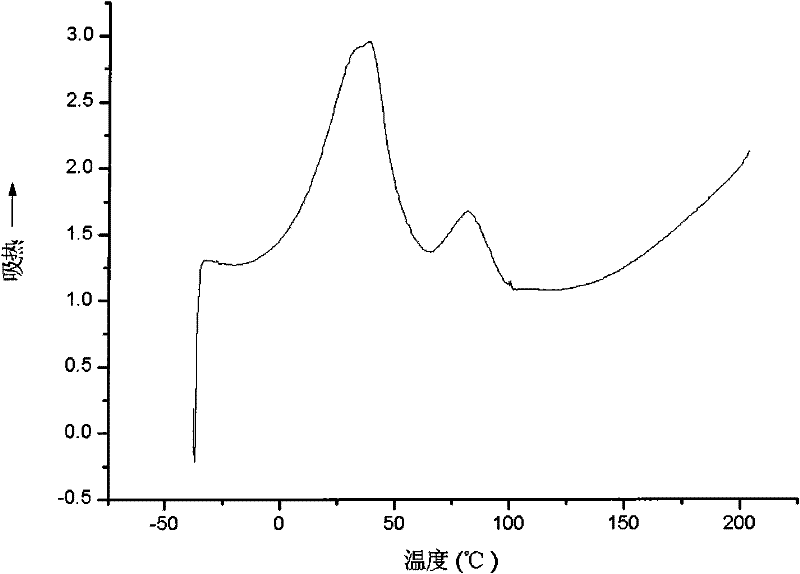
[0027] Dissolve 10 g of the cellulose savage ester prepared in Example 1 in chloroform, apply it evenly on the polished and derusted tinplate test piece with an applicator, and dry it naturally at a temperature of 25 ° C for 24 hours, and the coating film thickness is 25 to 40 Microns. The samples were then further cross-linked under UV light for 2 hours. The samples before cross-linking and after cross-linking were tested according to the standard GB / T 6739-1996 for the pencil hardness test of the paint film, according to the standard GB / T1732-93 for the impact strength test of the paint film, and for the paint film according to the standard GB 1720-79 Adhesion test, according to the standard GB 1731-93 to perform the paint film flexibility test. Scrape the samples before and after crosslinking from the tinplate sheet with a knife, prepare 10% tetrahydrofuran solution and filter them, and test the weight average molecular weight on the American waters 1515 gel chromatograph,...
Embodiment 3
[0031] 600 ml of the suction-filtered filtrate described in Example 1 was added into a 2000 ml round-bottomed flask, methanol was distilled off, and 590 ml of methanol was recovered. The remaining liquid is distilled to recover 1-N-n-butyl-3-trimethylimidazolium chloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com