Preparation method for 2-chloro-5-chloromethyl pyridine
A technology of chloromethylpyridine and a synthesis method is applied in the field of preparation of 2-chloro-5-chloromethylpyridine, and can solve the problems of inability to treat waste water or high treatment cost and high COD
Active Publication Date: 2011-01-19
NANKAI UNIV +1
View PDF11 Cites 16 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, there are obvious disadvantages in this synthetic route: 1. A large amount of N, N-dimethylformamide, referred to as DMF, is needed in the two steps of chlorination addition and cyclization, and every ton of 2-chloro-5-chloro The picoline needs to consume 1-1.2 tons of DMF; 2. A large amount of waste water is produced, 10 tons of high-concentration waste water is produced per ton of 2-chloro-5-chloropicoline, and the COD in the waste water is as high as 180,000-200,000 mg / liter
Wastewater contains a large amount of decomposition products of DMF and part of DMF, and also contains a large amount of phosphorus-containing compounds; 3. The generated wastewater cannot be treated or the treatment cost is extremely high
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
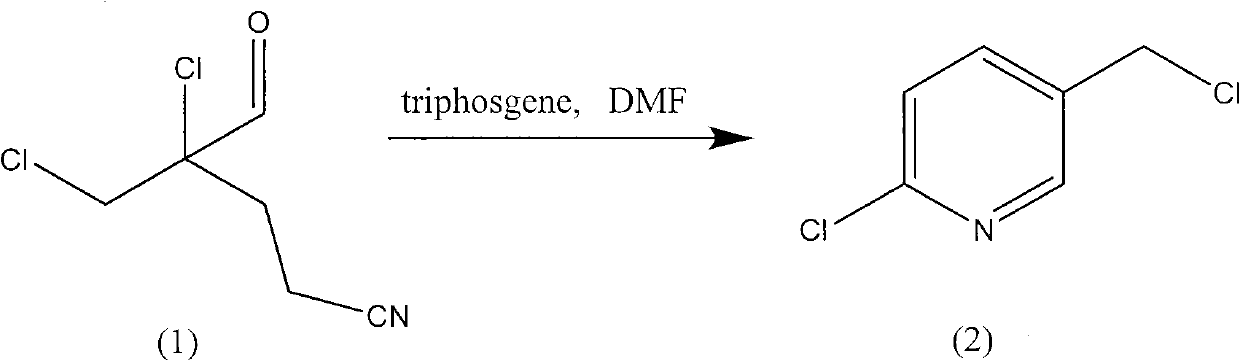
The invention relates to a synthesis method for 2-chloro-5-chloromethyl pyridine, in particular to an innovation on a chlorination addition and cyclization reaction. In the method, the insufficient of preparing the 2-chloro-5-chloromethyl pyridine in a cyclopentadiene-acrolein route is solved; the purpose of utilizing less DMF (Dimethyl Formamide) in the chlorination addition and the cyclization reaction is realized; solid triphosgene or diphosgene or phosgene are adopted to substitute phosphorus oxychloride and other phosphorous chlorination cyclization reagents, therefore, the problem of mass discharge of the DMF and phosphorous waste water in the production course is thoroughly solved; the production cost is reduced; and the cleaner production is realized.
Description
technical field The invention relates to a preparation method of 2-chloro-5-chloromethylpyridine. technical background 2-Chloro-5-chloromethylpyridine is an important intermediate of pesticides and pharmaceuticals. For example: 2-chloro-5-chloromethylpyridine is an important intermediate in the synthesis of important neonicotinoid insecticides imidacloprid, thiacloprid, acetamiprid, nitenpyram, etc., such as European patents EP247477, EP296453, EP685477 , EP235725, EP315826, EP192060, EP244777, EP0386565, EP580553, EP1031566, JP62292765, JP8259568, JP8291171, JP7242633, etc. There are many ways to synthesize 2-chloro-5-chloromethylpyridine. For example, 3-picoline oxidation, selective chlorination route, benzylamine-propionaldehyde route, morpholine-propionaldehyde route, cyclopentadiene-acrolein route. At present, 3-picoline oxidation and selective chlorination routes are mainly used in foreign countries. Most domestic manufacturers mainly use the cyclopentadiene-acrolei...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D213/61
CPCC07D213/61
Inventor 邹小毛李引红陈森傅翠蓉邹国亮
Owner NANKAI UNIV
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com