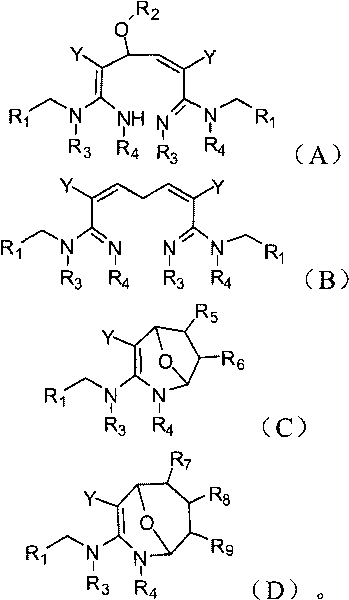
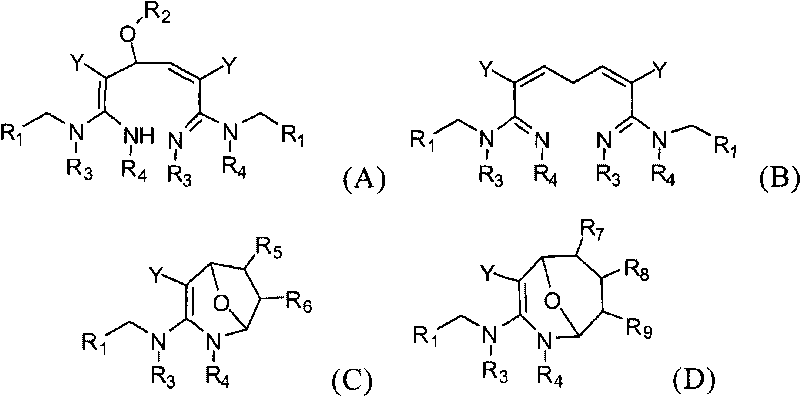
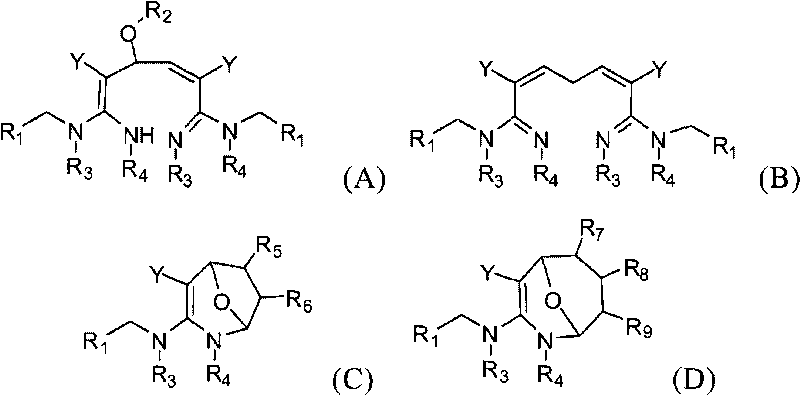
Dialdehyde-built disinsection activity-having nitrogen or oxygen-containing heterocyclic compound and preparation method
一种化合物、六元杂环的技术,应用在新型新烟碱类的杀虫剂领域,能够解决窄杀虫谱、限制用药选择性、制约化合物发展等问题,达到解决抗性问题、扩大杀虫谱的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The preparation method of the compound of the present invention
[0061] The compounds of the present invention can be synthesized by the reaction steps described above. Those skilled in the art can synthesize the compound of formula (a) in the reaction step according to the prior art literature, for example, refer to WO 2006056108A1, WO 2007101369A1 and PCT / CN2008 / 071115.
[0062] In a specific embodiment of the present invention, the synthetic method of formula (A) compound is as follows:
[0063] (R 2 for hydrogen).
[0064] In a specific embodiment of the present invention, the synthetic method of formula (B) compound is as follows:
[0065]
[0066] In a specific embodiment of the present invention, the synthetic method of formula (C) compound is as follows:
[0067]
[0068] In a specific embodiment of the present invention, the synthetic method of formula (C) compound is as follows:
[0069]
[0070] In one embodiment of the present invention, t...
Embodiment 1
[0104] Example 1 : 4-(1-((6-chloropyridin-3-yl)methyl)-4,5-dihydro-1H-imidazolidin-2-yl)-1-(1-((6-chloropyridine- Synthesis of 3-yl)methyl)imidazolidin-2-yl)-1,4-dinitro-3-buten-2-ol (compound 13)
[0105] Using 0.03mol of 2-chloro-5-chloromethylpyridine as a starting material, prepare 2-chloro-5-(2-nitromethylene-imidazolidine-1- Methyl)-pyridine, the yield was 56%; Rf=0.46 (petroleum ether: ethyl acetate=1:1); mp=156.9°C-161.8°C. GC MS (m / s) 220(25), 126(100), 90(9).
[0106] 4-(1-((6-chloropyridin-3-yl)methyl)-4,5-dihydro-1H-imidazolidin-2-yl)-1-(1-((6-chloropyridine-3 -base) Synthesis of methyl)imidazolidin-2-yl)-1,4-dinitro-3-buten-2-ol
[0107]
[0108]Add 1.27g (0.005mol) of 2-chloro-5-(2-nitromethylene-imidazolidin-1-ylmethyl)-pyridine, 30ml of anhydrous acetonitrile, and 3ml of 30% aqueous glyoxal In a 50ml round-bottomed flask, stir for half an hour, then add a catalytic amount of concentrated hydrochloric acid to catalyze, continue to stir, TLC to track...
Embodiment 2
[0110] Example 2 : 2-chloro-5-((-2-(-4-(1-((6-chloropyridin-3-yl)methyl)-4,5-dihydro-1H-imidazolidin-2-yl) Synthesis of -2-methoxy-1,4-dinitro-3-butenyl)imidazolidin-1-yl)methyl)pyridine (compound 14)
[0111]
[0112] 0.549g (0.001mol) of compound 1 was added to a 50ml round bottom flask, and then 10ml of methanol, 50ml of dichloromethane and a catalytic amount of concentrated hydrochloric acid were added, refluxed, and the reaction was tracked by TLC. After the reaction was finished, the solvent was removed, and the pure product was obtained as a yellow powder through column chromatography separation, with a yield of 62%.
[0113] mp=151.6-153.1°C; 1 H NMR (400Mz, DMSO-d 6 ): δ9.03(s, 1H), 8.38(d, J=2.0Hz, 1H), 8.36(d, J=2.0Hz, 1H), 7.81-7.85(m, 2H), 7.49-7.51(m, 2H), 6.50(d, J=7.2Hz, 1H), 5.35(d, J=15.2Hz, 1H), 5.19(d, J=15.2Hz, 1H), 4.80(d, J 1 =7.2Hz, 1H), 4.77(d, J=16.8Hz, 1H), 4.69(d, J=16.8Hz, 1H), 3.68(s, 3H), 3.88-3.95(m, 2H), 3.61-3.85 (m, 5H), 3.38-3.41 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com