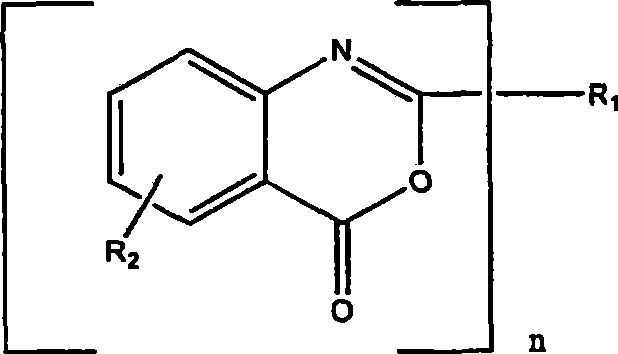
Process for preparing a benzoxazinone
A technology of benzoxazine and alkylimidazole, which is applied in the field of preparation of benzoxazine, can solve the problems of increasing the complexity of the method, generating additional costs, loss of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]125gm Technical Grade* Itatoic Anhydride (ISA) was dissolved in 1475gm N-Methylimidazole (NMI), the mixture was heated to 60°C and 91.3gm Terephthaloyl Chloride (TPC) was added over 30 minutes with good stirring. The temperature was raised to 120 to 130°C and maintained at this temperature for a further 4 hours. The mixture was cooled to 30°C and the precipitate was filtered. The pellet was reslurried in 400 ml isopropanol (99%) (IPA) and washed 3 times with 150 ml IPA. The resulting precipitate was reslurried in 450 gm NMI heated to 95°C, kept under stirring for 30 minutes and filtered. The wet cake was washed with hot IPA and dried under vacuum (20mm) at 80°C.
[0044] The product 107 gm [2,2-p-phenylene-bis(3,1-benzoxazin-4-one)] was a white powder and was 98.5% pure by HPLC analysis. The product was extracted using dimethylformamide (DMF), and the extract was found to have a color value of <50 APHA.
[0045] * Technical grade itatoic anhydride (sold in Europe an...
Embodiment 2A
[0047] Dissolve 32.6 gm of isatoic anhydride (ISA) (0.2 mol) in a mixture of dimethylformamide (DMF) (150 mol) and 18.2 gm (0.22 mol) of N-methylimidazole (NMI) at 86 to 90°C middle. At this temperature, 21.7 gm (0.107 mol) of terephthaloyl chloride (TPC) were added to the mixture within 30 minutes with stirring. The temperature of the reaction mixture was raised to 110 to 120°C and stirred for 4 hours. The mixture was cooled to 20 to 25°C and stirred for a further 2 hours. Subsequently, the product was filtered and washed on the filter with 150 mL of methanol and dried at 60°C. The yield of 2,2-p-phenylene-bis[4H-3,1-benzoxazin-4-one] / (PBBOX) was 29.8 gm (81%).
Embodiment 2B
[0048] Embodiment 2B (reference example)
[0049] In the reaction of itatoic anhydride (0.2mol), DMF (150mL), pyridine (0.22mol) and TPC (0.107mol) under the above reaction conditions, the yield of PBBOX was only 45.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com