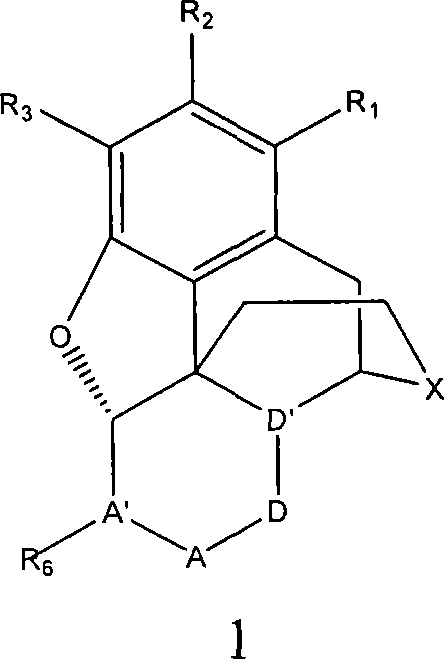
Processes for preparing morphinans and intermediates thereof
一种烷基、去甲二氢蒂巴因酮的技术,应用在有机化学等方向,能够解决产率和效率降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
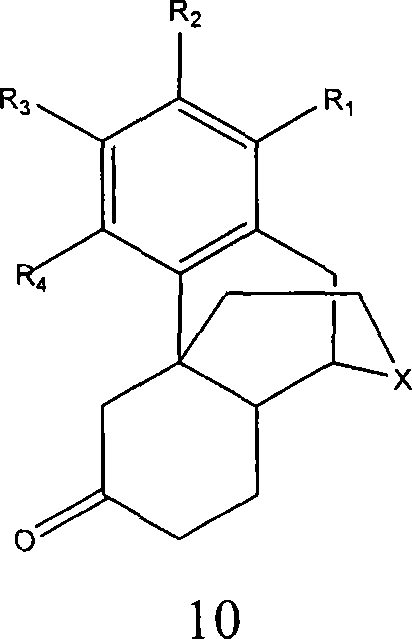
[0246] Conversion of α,β-Hexahydroisoquinolones to Protected β,γ-Hexahydroisoquinolones
[0247]
[0248] The isomer 1-(2′-bromo-4′-methoxy-5′-hydroxybenzyl)-2-formyl-1,3,4,7,8-hexahydroisoquinoline-6- Ketone and 1-(2'-bromo-4'-methoxy-5'-hydroxybenzyl)-2-formyl-1,3,4,5-hexahydroisoquinolin-6-one in chloroform solvent The solution in is added to ethylene glycol (such as a keto-protected compound) within 1 hour to form 1-(2'-bromo-4'-methoxy-5'-hydroxybenzyl)-2-formyl-6 - Ketal - 1,3,4,5,7,8-Hexahydroisoquinoline solution (>90% area / area by HPLC analysis). solution was added to 5% NH 4 OH (200 mL), a suspension formed and extracted with chloroform (3 x 40 mL). The combined organic layers were washed with water (3 x 100 mL) and placed under vacuum until dry to give the product as a white solid (1.03 g, 80% area / area and 70% w / w by HPLC).
Embodiment 2
[0250] Hydrolysis of protected β, γ-hexahydroisoquinolones to give unprotected β, γ-hexahydroisoquinolones
[0251]
[0252] 1-(2'-Bromo-4'-methoxy-5'-hydroxybenzyl)-2-formyl-6-ketal-1,3,4,5,7,8-hexahydroisoquinone A sample of morphine (0.97 g) was dissolved in 10 mL of 88% acetic acid. The resulting solution was stirred at room temperature for 1 hour. After the solution was stirred for 1 hour, the solution was washed with 50 mL H 2 Dilute with 0 and 40mL chloroform, swirl, and then pour into a 500mL separatory funnel. After the mixture was combined and separated, the organic layer was separated. An additional 40 mL of chloroform was added to the aqueous layer. The solution was repeatedly mixed and separated, and the organic layer was separated again to obtain an aqueous layer in a separatory funnel. Another 40 mL of chloroform was added to the aqueous layer. The solution was swirled again, and after the organic layer was separated again, an aqueous layer was obtained...
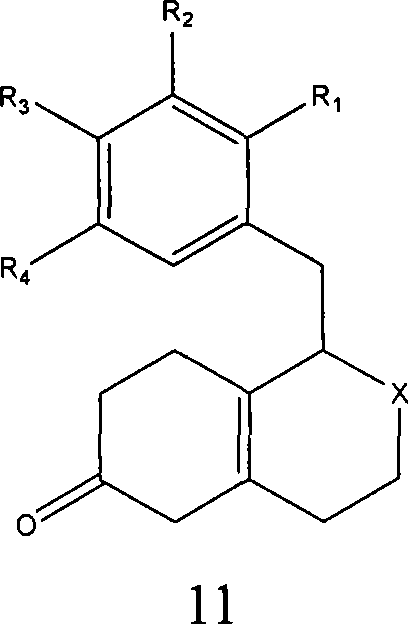
Embodiment 3
[0254] Grewe cyclization step
[0255]
[0256] A mixture of 270 mL of 98% triflic acid and 60 mL of 99% triflic anhydride was added to a 2 L round bottom flask. The mixture was heated to reflux, and the steam temperature reached 88°C-130°C. solution in N 2 Cool down to 5°C-10°C. Dissolve 100 g in 750 mL of CHCl 3 1-(2′-bromo-4′-methoxy-5′-hydroxybenzyl)-2-formyl-1,3,4,5,7,8-hexahydroisoquinoline-6- The ketone (eg, β,γ-hexahydroisoquinolinone) was placed in a 1 L round bottom flask under nitrogen. The β,γ-hexahydroisoquinolinone solution was added to the triflic acid solution at a constant rate over 30 minutes with constant stirring. The trifluoromethanesulfonic acid solution was surrounded by an ice bath to maintain the solution temperature at 15°C during the addition of the β,γ-hexahydroisoquinolinone. After the combined solutions were complete, the reaction was warmed to room temperature and stirred for approximately 12 hours. After stirring at room temperature, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com