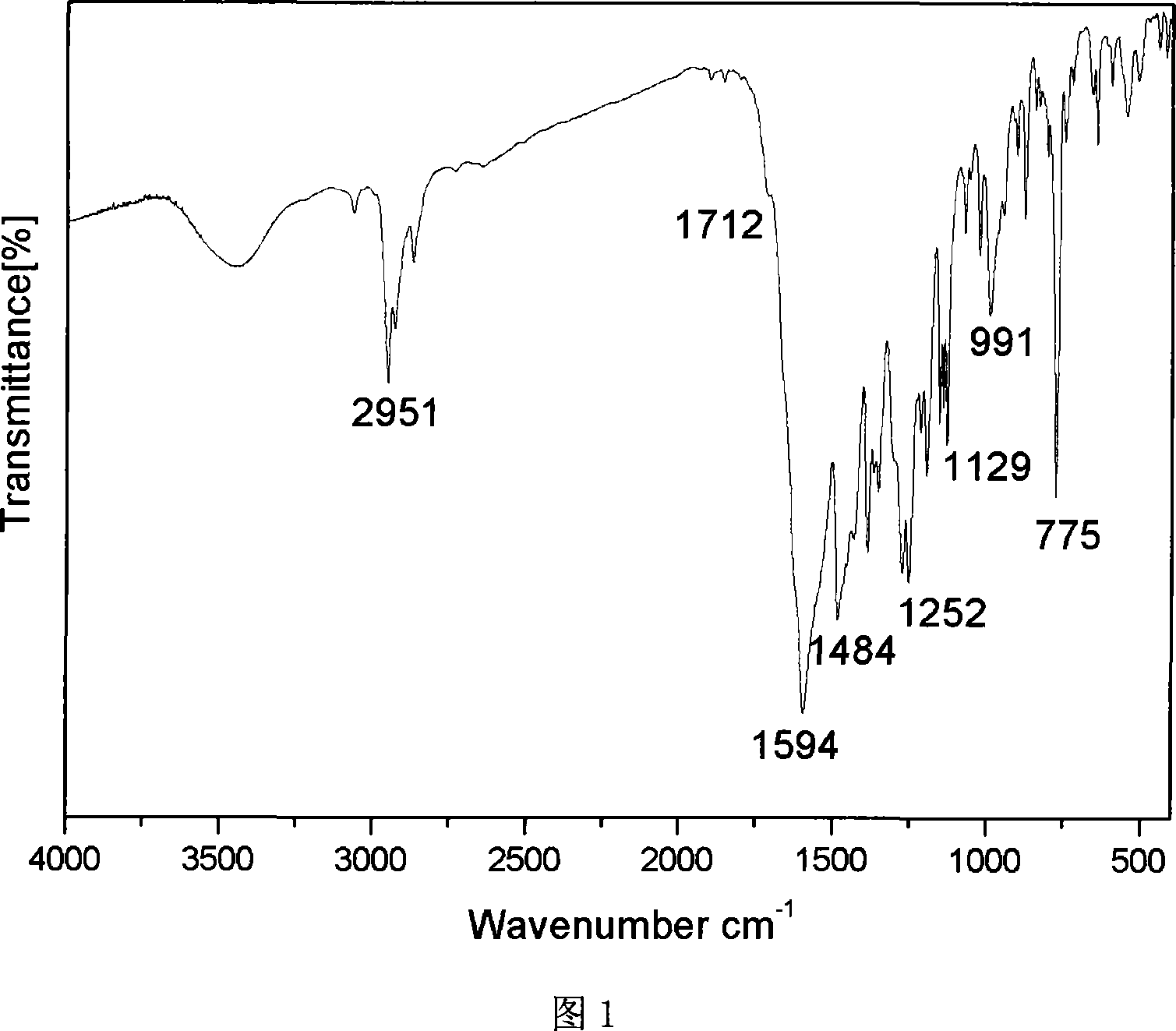
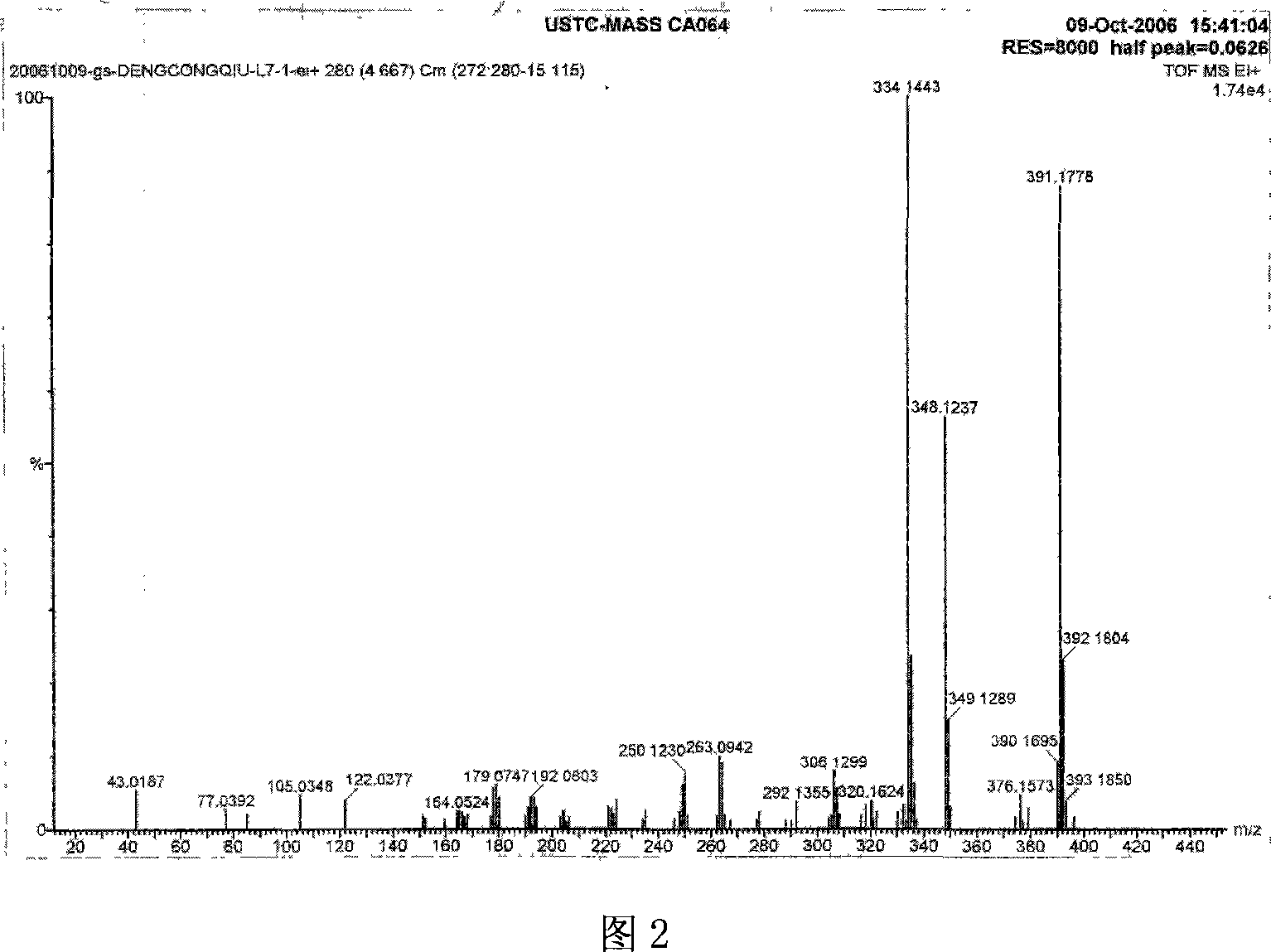
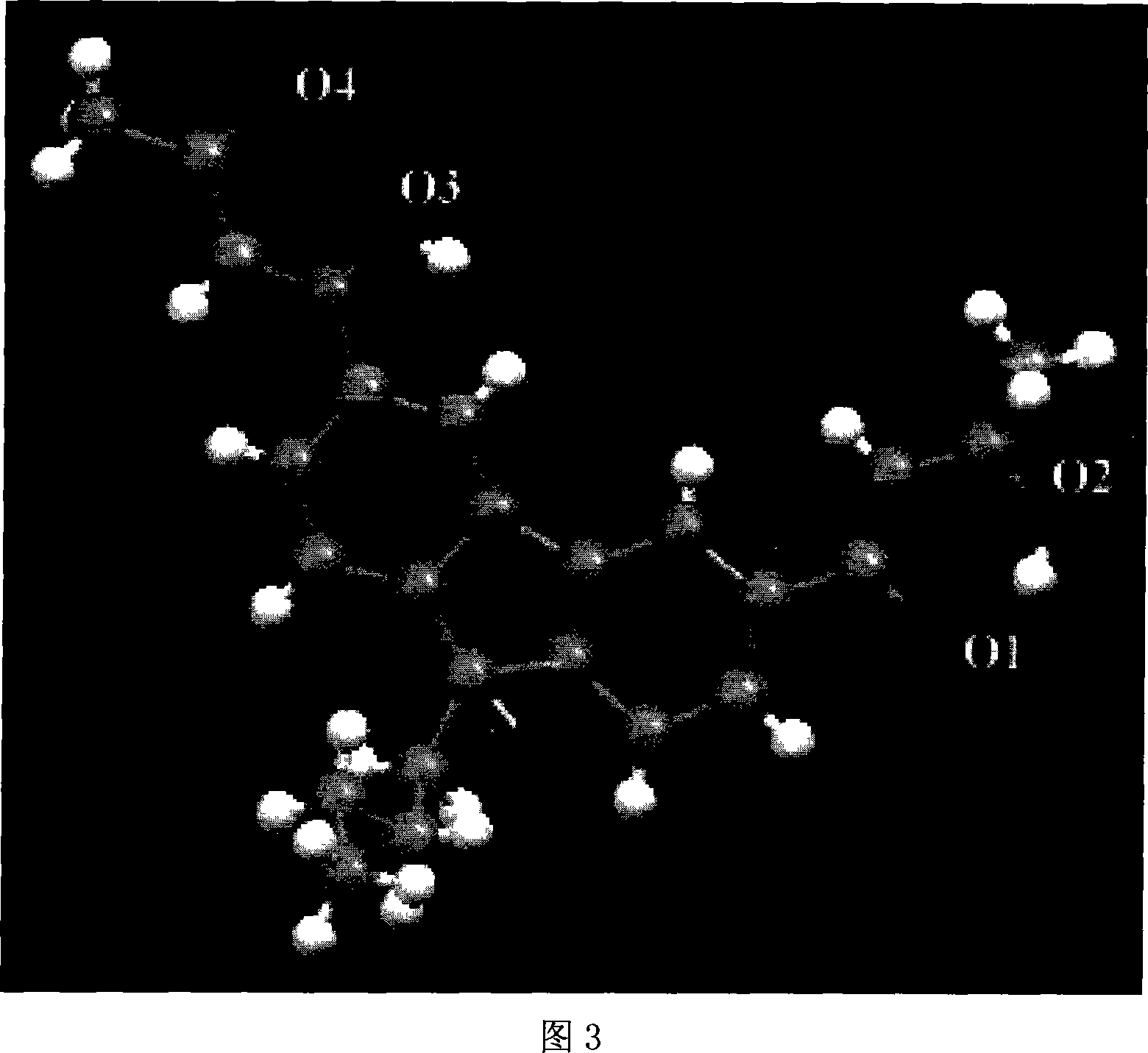
Carbazolyl-functional bi-beta-diketo derivative and its production
A technology of functionalization and derivatives, applied in the field of carbazole-functionalized bis-β-diketone compounds, 9-n-butyl-3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] synthetic part
[0038] 1. Synthesis of N-n-butylcarbazole (BCz)
[0039] Pour 7.52 g of carbazole and 4.4 g of NaOH into a 150 ml round bottom flask, add 60 ml of acetone, stir, and the temperature rises to 60°C to reflux. After about 10 minutes, add 5 drops of Aliquid336, and then reflux for 2 hours, then add 5.5ml of n-bromobutane, and continue to reflux for 24 hours. Replace the device with a distillation device to evaporate the acetone as much as possible. Pour the remaining reactants into a 1000mL beaker filled with ice-water mixture, stir vigorously with a large magnetic stirring bar, and a large amount of yellow solids precipitate out. After stirring for 30 minutes, put it in the refrigerator for 3 to 4 hours, and filter it with suction. A crude product of 8.03 g was obtained. Recrystallized with absolute ethanol to obtain 7.89 g of white needle-like crystals, with a yield of 91.6%.
[0040] 2. Synthesis of 3,6-diacetyl-N-n-butylcarbazole (BCB)
[0041] Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com