Method for separating and determining pitavastatin and its optical isomer by means of liquid chromatography
A technology for optical isomers and determination methods, applied in the field of analytical chemistry, can solve unreliable problems, achieve the effects of improving symmetry, ensuring stability, and controlling quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Instruments and Conditions
[0032] U.S. Agilent 1100 high-performance liquid chromatography system and workstation; automatic sample injection; with CHIRALPAK-AD chiral chromatography column (250mm × 4.6mm) as the separation column; ultraviolet detection wavelength: 245nm; mobile phase: n-hexane-ethanol solution (containing 1.0% trifluoroacetic acid) (92:8) as mobile phase; column temperature 40°C. The injection volume was 10 μl.
[0033] Experimental procedure
[0034] Take about 25mg of pitavastatin calcium racemate, put it in a 50ml measuring bottle, add ethylene glycol dimethyl ether to dissolve and dilute to the mark, shake well, and use it as the test solution.
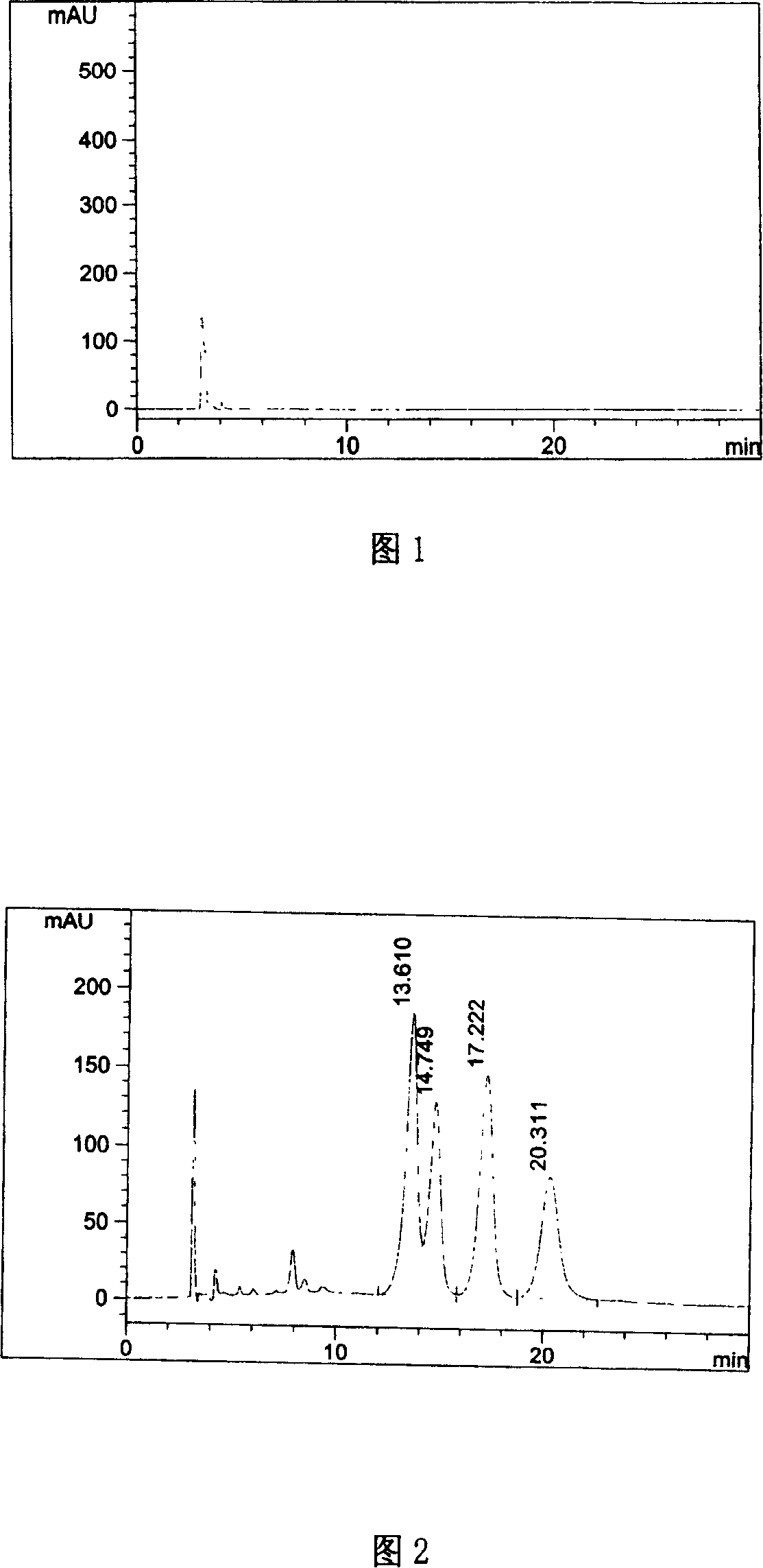
[0035] Respectively take reagent blank solution and need testing solution, carry out high performance liquid chromatography analysis according to above-mentioned conditions, record chromatogram, the result is shown in Fig. 1, Fig. 2.
[0036] The chromatographic peak of retention time 17.222 minutes i...
Embodiment 2
[0038] Take about 25mg of pitavastatin calcium, put it in a 50ml measuring bottle, add ethylene glycol dimethyl ether to dissolve and dilute to the mark, shake well, and use it as the test solution.
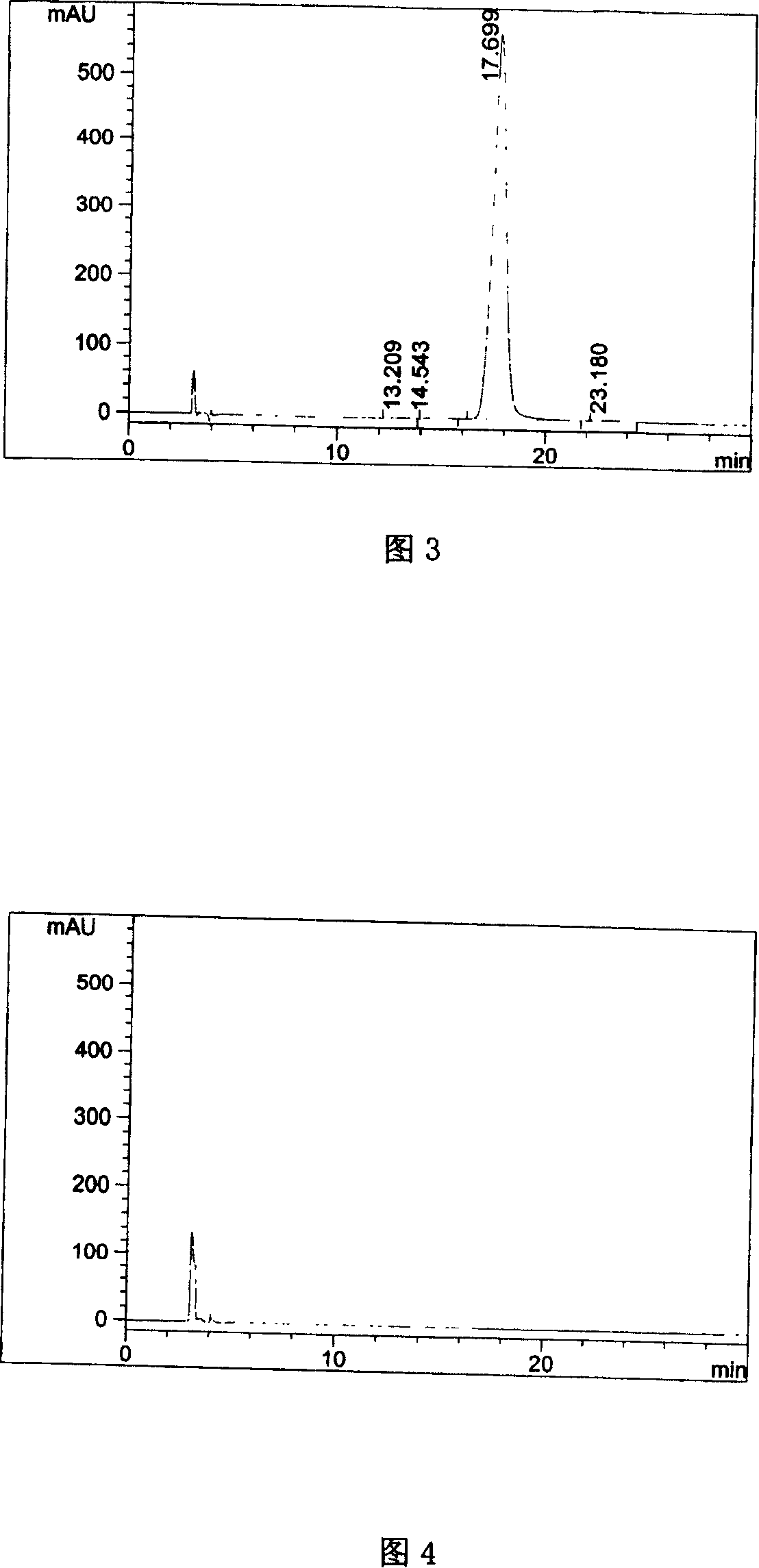
[0039] Get need testing solution, carry out high performance liquid chromatography analysis according to the condition of embodiment 1, record chromatogram, the results are shown in Fig. 3.
[0040] Figure 3 proves that the optical purity of pitavastatin calcium meets the requirements of raw materials, and this method can be used for quality monitoring of pitavastatin calcium.
Embodiment 3
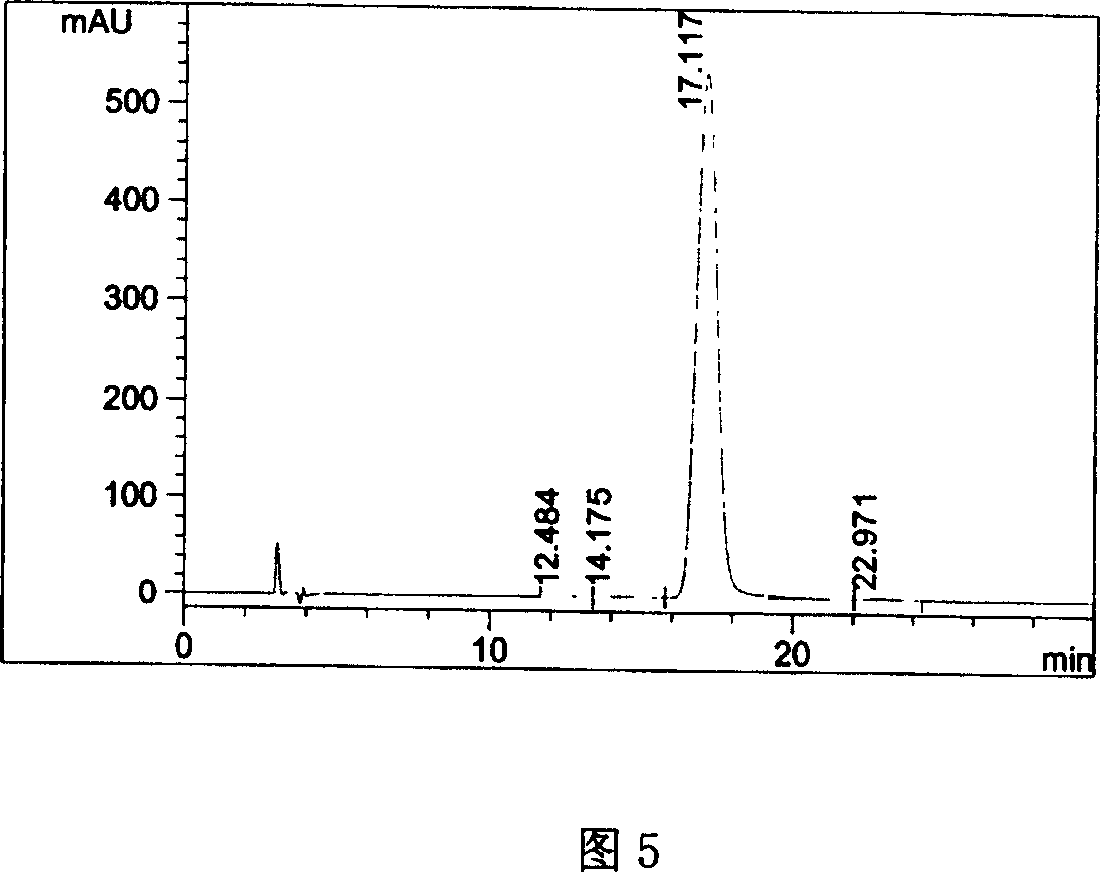
[0042] Take an appropriate amount of pitavastatin calcium tablets, approximately equivalent to 25 mg of pitavastatin calcium, put it in a 50ml measuring bottle, add an appropriate amount of ethylene glycol dimethyl ether, shake to dissolve, dilute to the mark with ethylene glycol dimethyl ether, and shake well , filtered, and the filtrate was used as the test solution. Get need testing solution, carry out high performance liquid chromatography analysis according to the condition of embodiment 1, and carry out adjuvant blank test with method, the results are shown in Fig. 4, Fig. 5.
[0043] Figure 4 proves that the adjuvant blank does not interfere with the determination, and Figure 5 shows that this method can be used for quality monitoring of preparations containing pitavastatin calcium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com