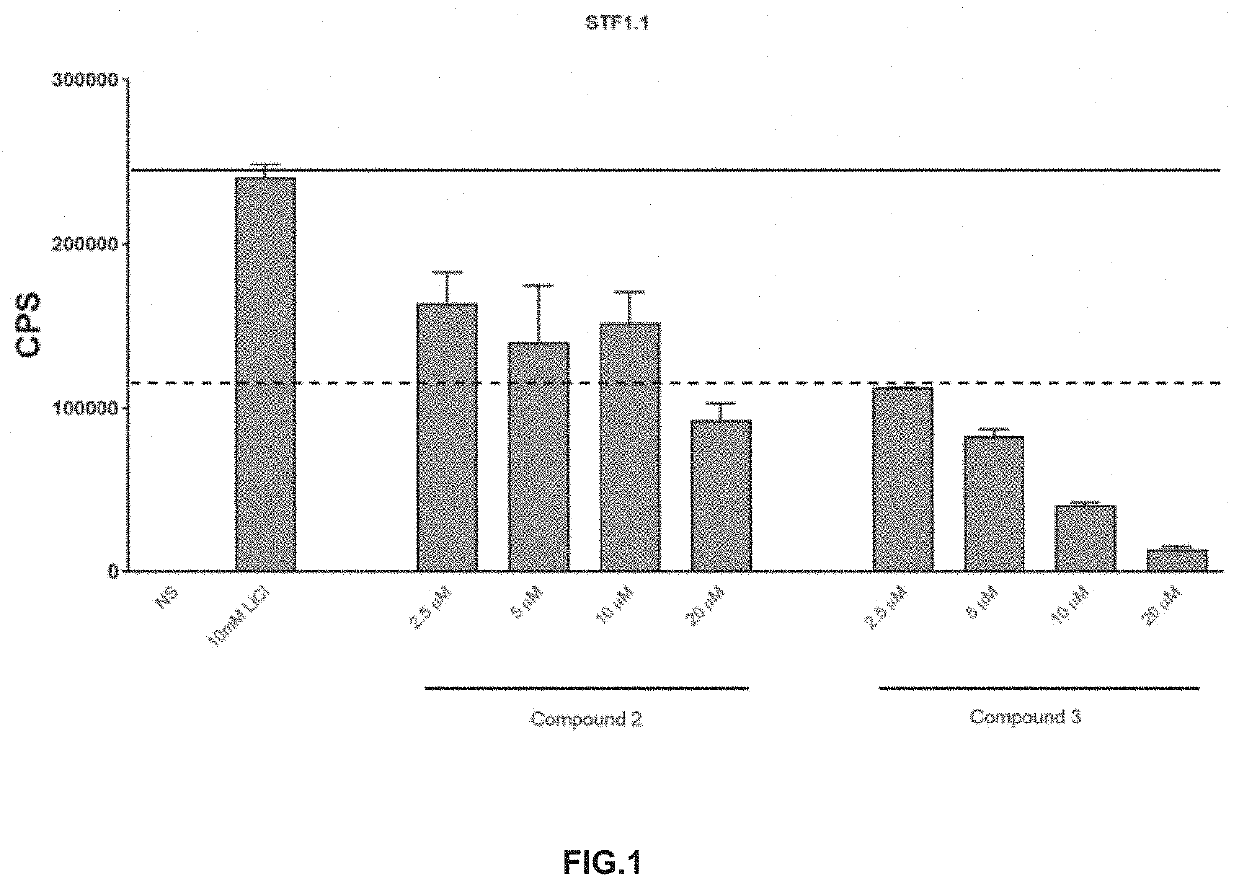
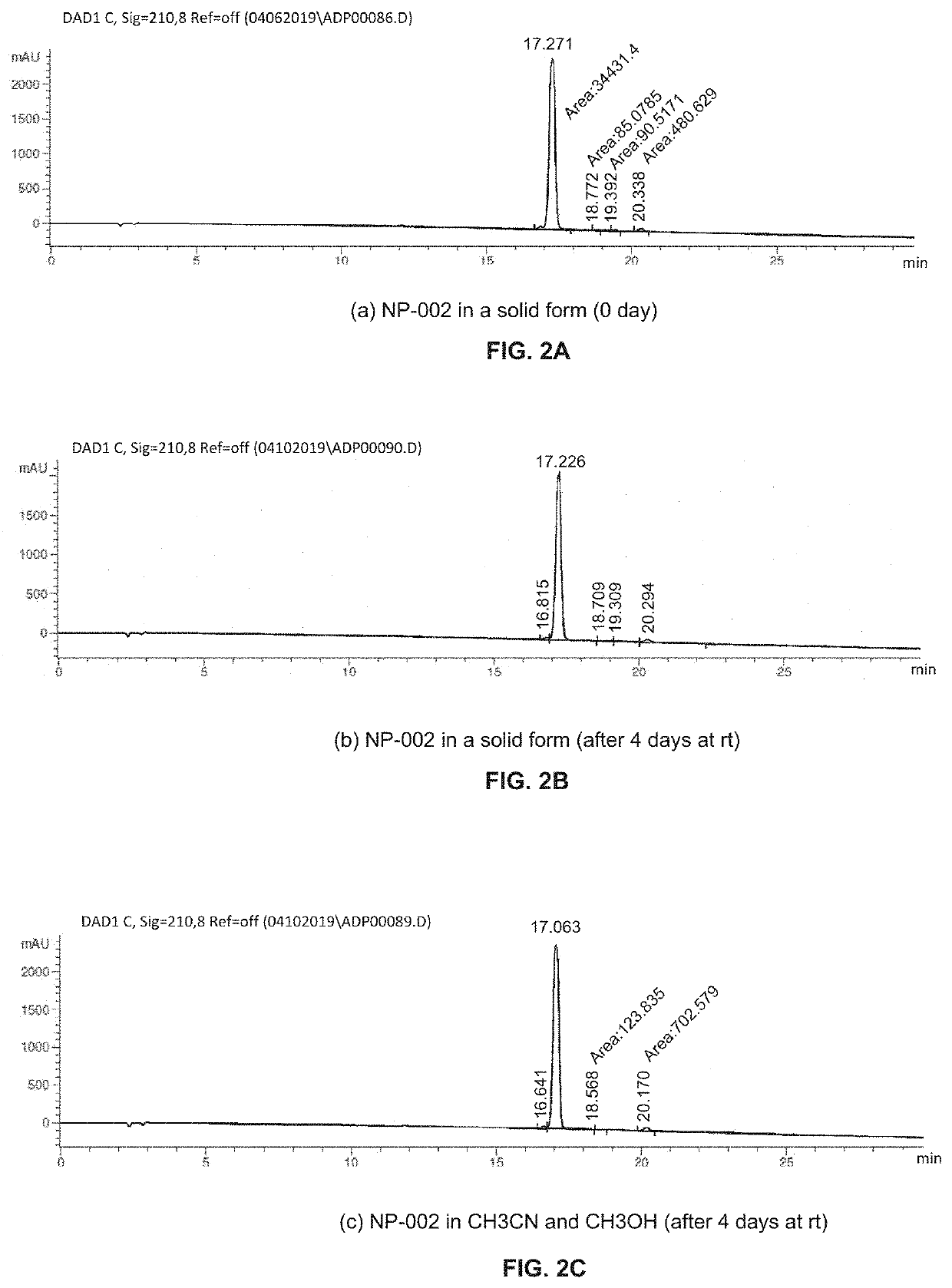
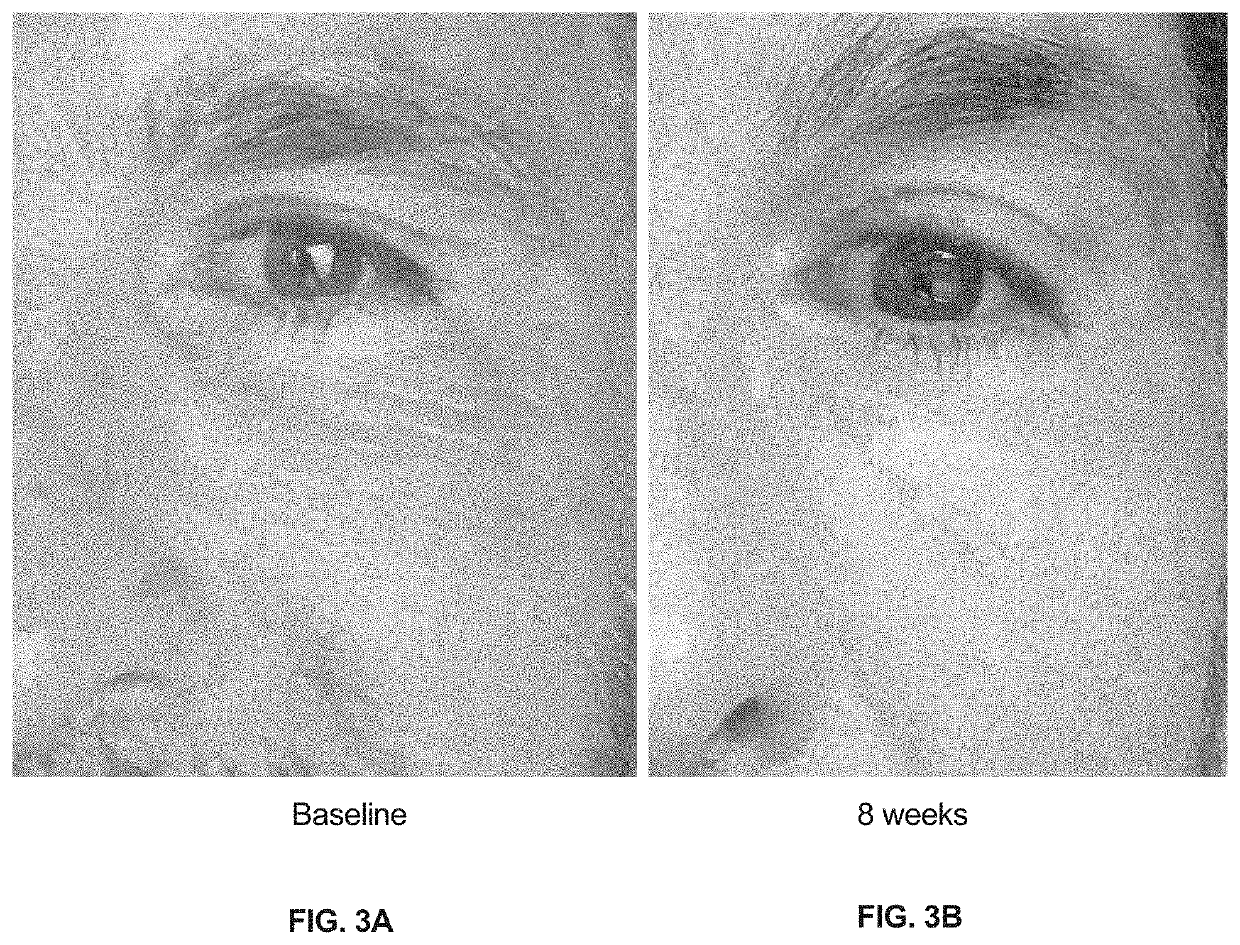
Topical agents for dermatological applications
a dermatological and topical agent technology, applied in the field of dermatological applications, can solve the problems of cosmetic skin improvement and hair growth, not speeding up the healing process, and having a negative effect on the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
working examples
[0105]An illustration of the preparation of exemplary compounds of the present invention is provided in the representative Examples and Schemes below, wherein specific non-limiting examples of compounds are intended to illustrate particular embodiments of the present invention, and are not intended to limit the scope of the specification or the claims in any way. The compounds of the present invention may be prepared by the synthetic sequence shown in the non-limiting Examples and Schemes below. A skilled artisan will appreciate that other routes of synthesis may be employed as well. In particular, other routes of synthesis may in fact be applied to certain aspects of the present invention. For example, (R)-3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-4-(tert-butyldimethylsilyloxy)butanoic acid can be prepared according to Step 1-2 of Example 3, and used for the synthesis of compound 2. The skilled artisan is referred to general textbooks, such as March's Advanced Organic Chemistry (...
example 1
[0106](Reagents, synthetic methods, and biological characterization assays used) Unless otherwise noted, all reagents, starting materials and solvents were obtained from commercial suppliers and used without further purification. Concentration or evaporation refers to evaporation under vacuum using a Buchi rotatory evaporator.
[0107]Reaction products were purified by silica-gel chromatography with the solvent system indicated, or by HPLC purification using a C18 reverse phase semi-preparative HPLC column with solvent A (0.1% of TFA in water) and solvent B (0.1% of TFA in CH3CN) as eluents. All final products have at least 95% purity as determined by analytical HPLC analysis with UV detection at 210 nm and / or 254 nm. Reported yields are isolated yields.
[0108]Analytical HPLC analysis was performed on an Agilent 1100 HPLC with a Phenomenex Luna C18 (2) column (3 micron, 150×4.6 mm id) at a flow rate of 0.6 mL / min, eluting with a binary solvent system A and B using a 35%-70% B in 20 min ...
example 2
(Synthesis of (S)-2-amino-3-(4-tert-butoxyphenyl)-N-(2,2-diethoxyethyl)-N-(naphthalen-1-ylmethyl)propanamide (1))
[0113]Compound 1 was prepared according to the procedures disclosed in U.S. Pat. No. 7,671,054, as shown in Scheme 1, starting from 1-naphthaldehyde. The title compound 1 as a colorless oil. MS (ESI): m / z 493.3 (M+H)+; analytical HPLC: 15.3 min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| elasticity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com