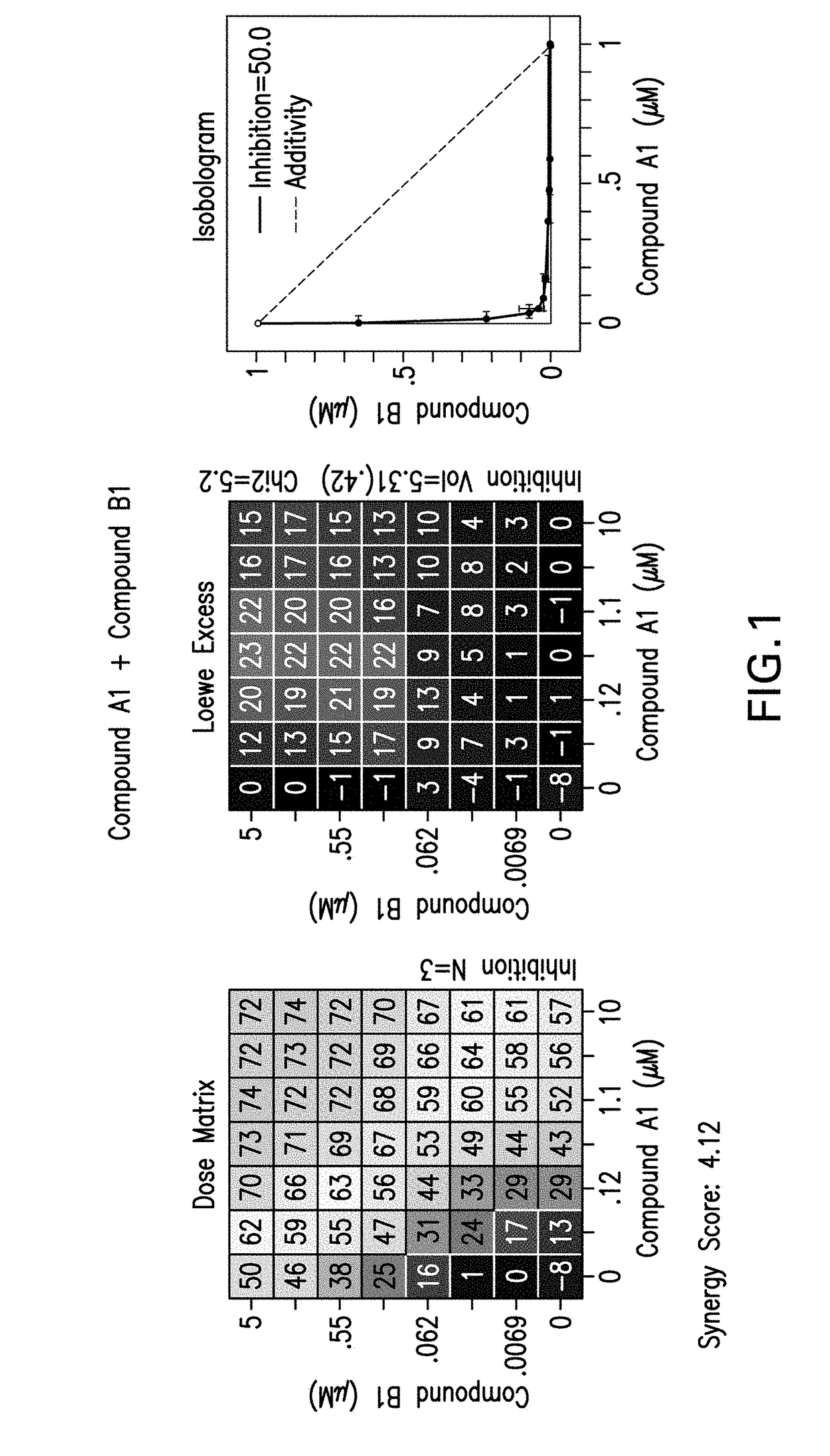
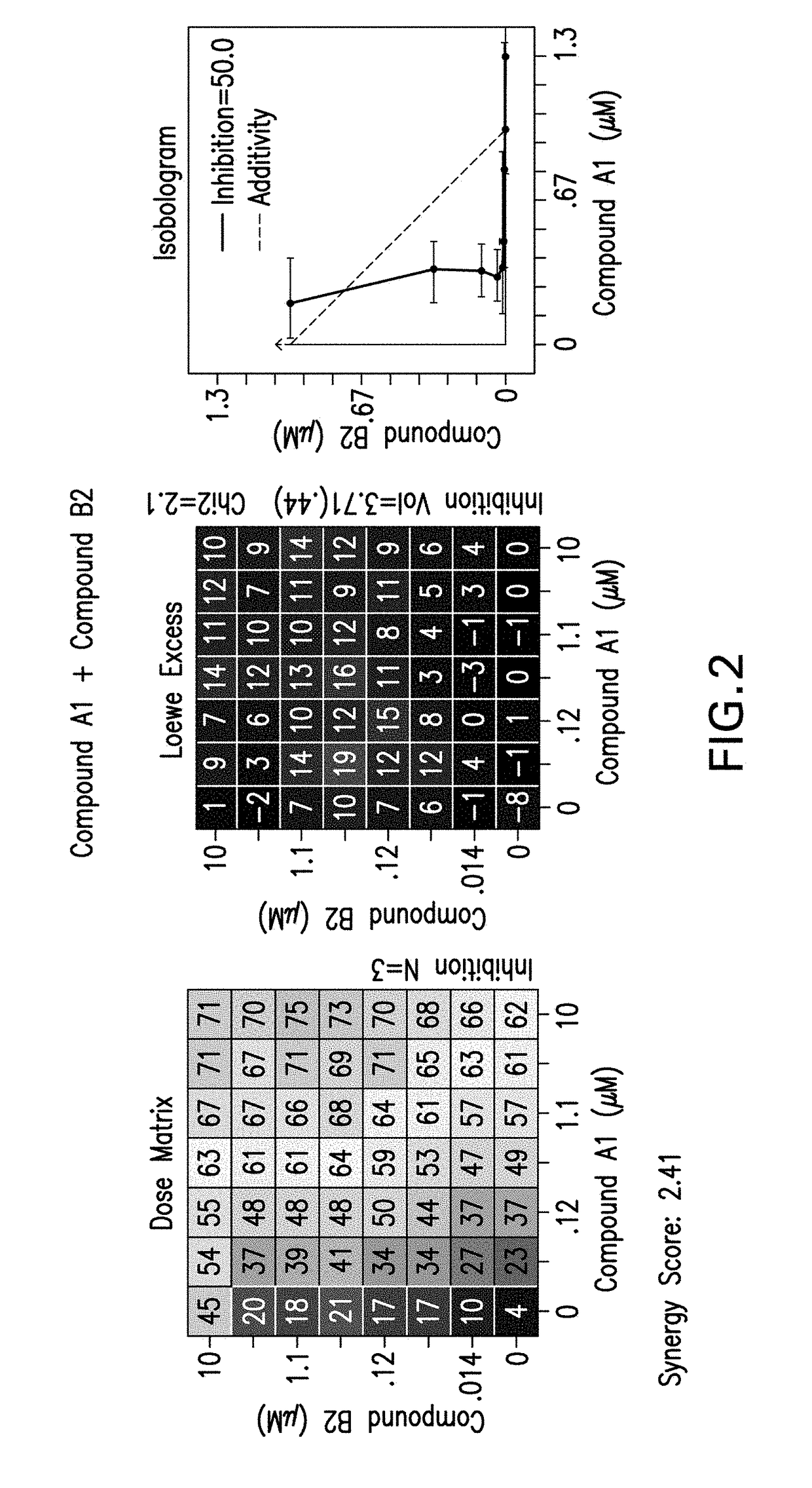
Combination therapy for the treatment of cancer
a cancer and combination therapy technology, applied in the field of combination therapy, can solve the problems of abnormal cell metabolism, poor patient prognosis, and deregulation of cell proliferation, and achieve the effect of reducing the dose needed and increasing the maximum level of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0235]The following experimental procedure is performed to demonstrate the efficacy and anti-proliferative activity of Compound A1 in double or triple combination in the treatment of breast cancer:
Preparation of Compounds / Reagent Solutions
[0236]Compound A1 (a CDK4 / 6 inhibitor, 10 mM), Compound B1 (Letrozole, Sigma, 10 mM), Compound B3 (Fulvestrant, Sigma, 10 mM), Compound B2 (Exemestane, Sigma, 10 mM), Compound C1 (a PI3K inhibitor, 10 mM), Compound C3 (an mTor inhibitor, 10 mM) and Compound C2 (a PI3K inhibitor, 10 mM) were dissolved in DMSO. Δ4A (the precursor androstenedione 10 mM) were dissolved in ethanol. All these reagents were stored in aliquots at −20° C.
Cell Culture
[0237]MCF7 human breast carcinoma cells were provided by Dr. Chen Shivan (City of Hope National Medical Center, CA, USA), which were stably transfected with the aromatase expression vector bearing the neomycin (G418) resistance gene (also named MCF7 / Aro). Aromatase converts the precursor androstenedione (Δ4A) in...
example 2
[0264]The following experimental procedure is performed to demonstrate the efficacy and anti-proliferative activity of Compound A2 or Compound A3 in double or triple combination in the treatment of breast cancer:
Preparation of Compounds / Reagent Solutions
[0265]Compound A2 (a CDK4 / 6 inhibitor, 10 mM), Compound A3 (a CDK4 / 6 inhibitor, 10 mM), Compound B1 (Letrozole, Sigma, 10 mM), Compound B3 (Fulvestrant, Sigma, 10 mM), Compound B2 (Exemestane, Sigma, 10 mM), Compound C1 (a PI3K inhibitor, 10 mM), and Compound C3 (an mTor inhibitor, 10 mM) were dissolved in DMSO. Δ4A (the precursor androstenedione, 10 mM) were dissolved in ethanol. All these reagents were stored in aliquots at −20° C.
Cell Culture
[0266]MCF7 human breast carcinoma cells were provided by Dr. Chen Shivan (City of Hope National Medical Center, CA, USA), which were stably transfected with the aromatase expression vector bearing the neomycin (G418) resistance gene (also named MCF7 / Aro). Aromatase converts the precursor andro...
example 3
[0300]A clinical trial is currently on going to further the clinical development of the two investigational agents in ER+ breast cancer, Compound A1 (CDK4 / 6 inhibitor) and Compound C1 (PI3K inhibitor). This is a multi-center, open-label, dose finding Phase Ib / II trial. The Phase Ib part is a three-part dose escalation study to estimate the MTD and / or RP2D for two double combinations: Compound A1 with letrozole and Compound C1 with letrozole followed by estimation of the MTD and / or RP2D of the triple combination of Compound A1+Compound C1 with letrozole.
[0301]The three-part Phase Ib will be followed by a randomized Phase II study to assess the preliminary anti-tumor activity of the two double combination regimens (Compound A1+letrozole and Compound C1+letrozole) versus the triple combination (Compound A1+Compound C1 with letrozole) and to further evaluate their safety in patients with ER+ / HER2− locally advanced or metastatic breast cancer.
[0302]Approximately 290 adult women with ER+ / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com