Method and Apparatus for Verification of Clinical Trial Adherence
a clinical trial and verification method technology, applied in the field of patient compliance in clinical drug trials, can solve problems such as personal liability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
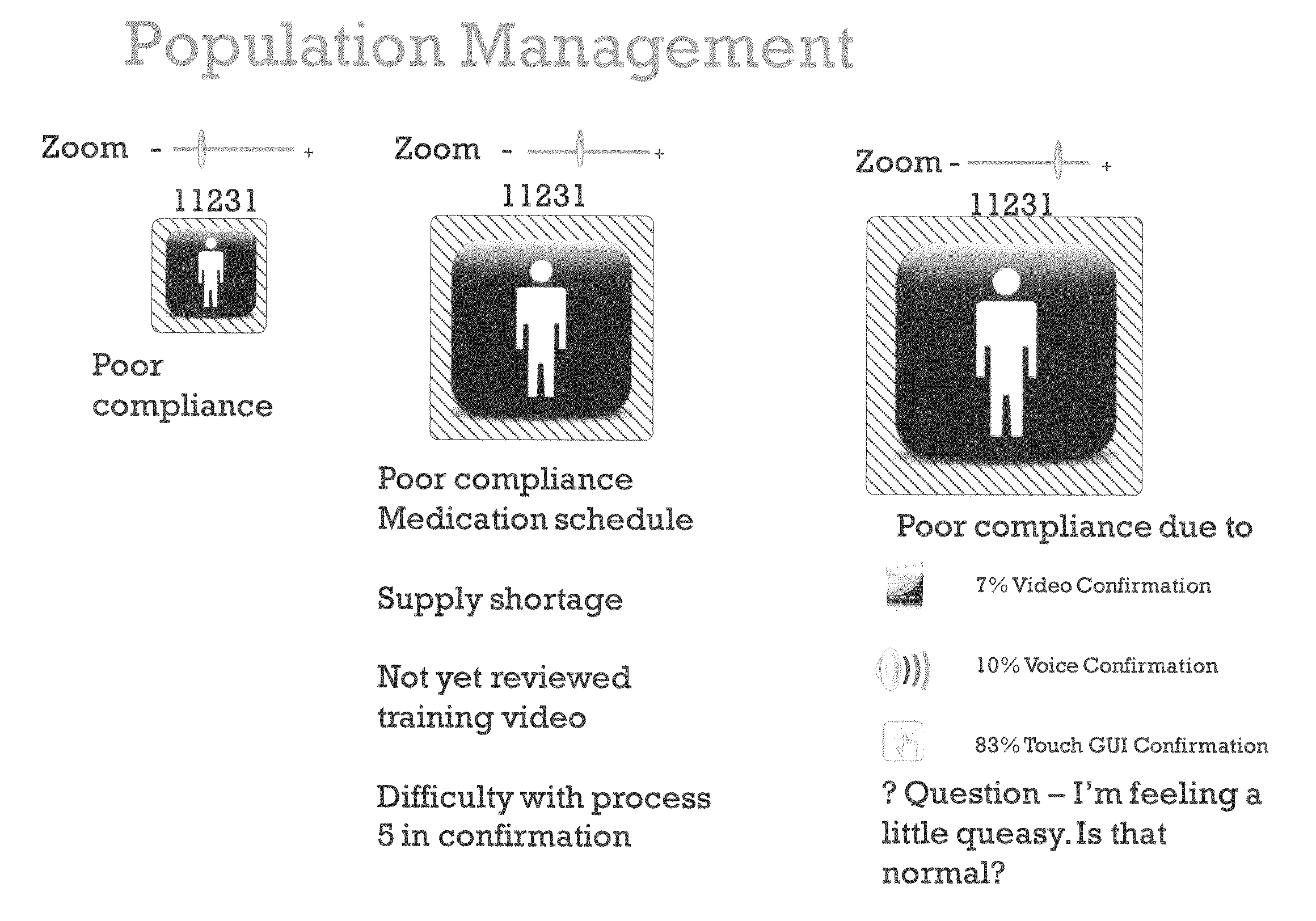
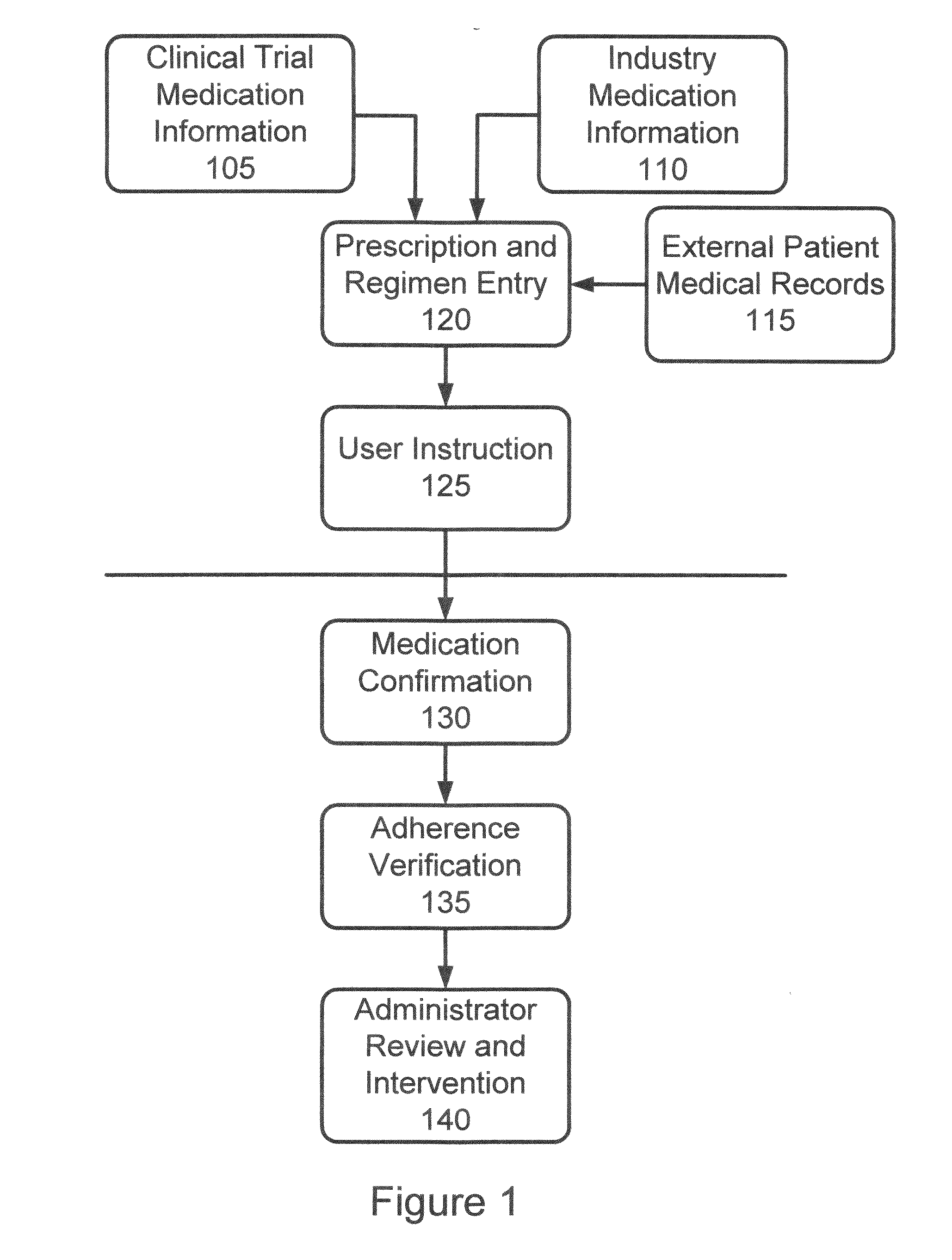
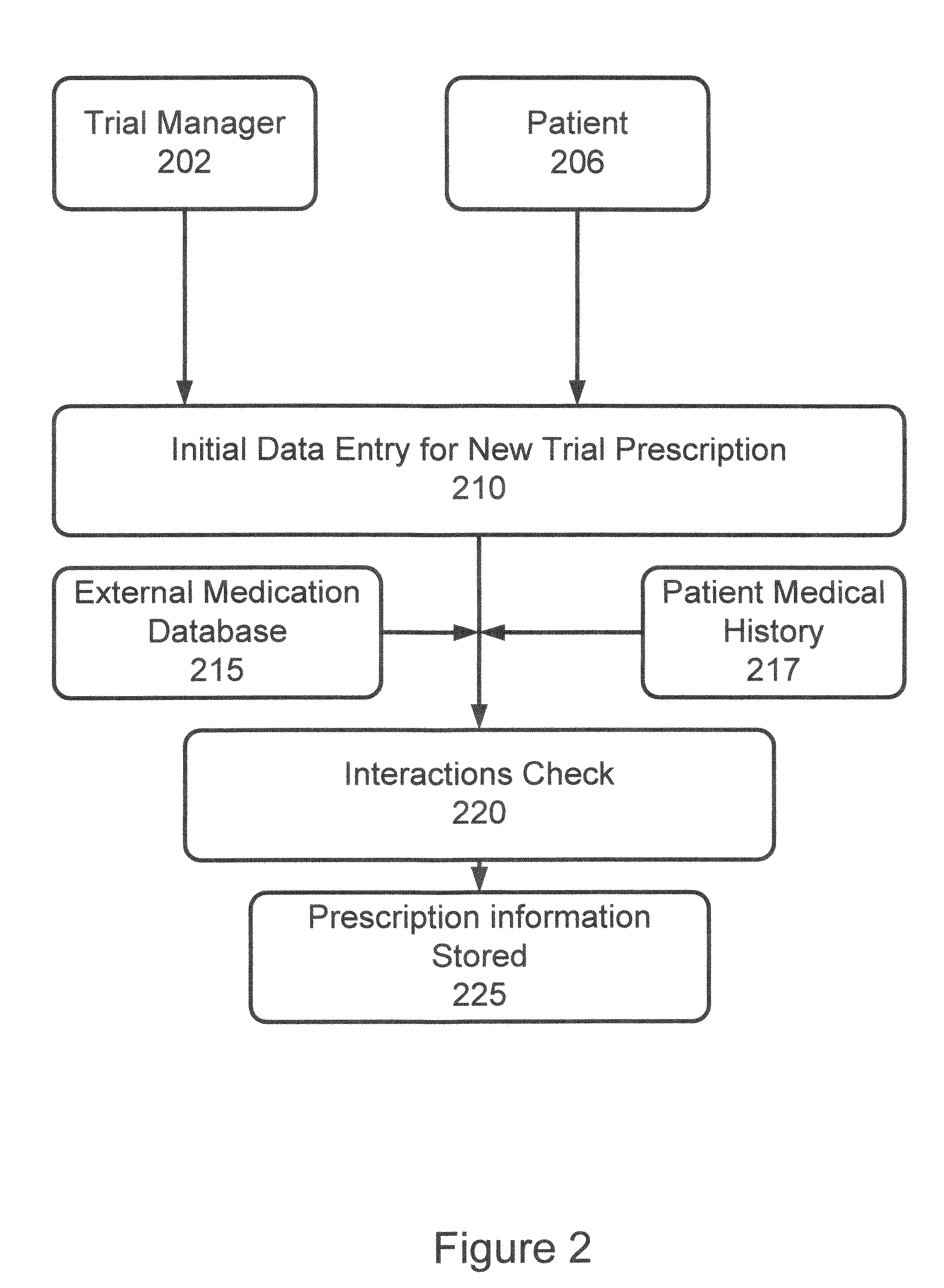
[0037]In accordance with the invention, a system and process are provided that improve adherence to medical protocol in a clinical trial setting, and give administrators a tangible and concrete manner in which to confirm compliance or lack thereof, and the ability to intervene early in the process to ensure that patients enrolled in such a clinical trial study are properly taking their medication. The system and method of the invention provide for instructions to patients on the use of the prescription medication under trial, verification to a doctor or other service provider of patient adherence to the prescribed protocol, and statistical and individual analysis of adherence rates to ensure proper clinical trial administration. The system and method further improve a level of care received by a patient in a clinical trial, and other setting, as well as improve the perception of that level of care by the patient. The system and method of the invention further provide the ability to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com