Compositions and methods comprising serine protease variants
a serine protease and variant technology, applied in the field of protein engineering and serine protease variants, can solve the problems of laborious and time-consuming most methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Assays
[0236]In the following Example, various assays were used as set forth below for ease in reading. Any deviations from the protocols provided below are indicated.
A. TCA Assay for Protein Content Determination in 96-Well Microtiter Plates (“TCA Assay”)
[0237]For BPN′ (e.g., a reference protease) and variants thereof, this assay was started using filtered culture supernatant from microtiter plates grown 3-4 days at 33° C. with shaking at 230 rpm and humidified aeration. A fresh 96-well flat bottom microtiter plate (MTP) was used for the assay. First, 100 μL / well of 0.25 N HCl was placed in each well. Then, 50 μL of filtered culture broth was added. The light scattering / absorbance at 405 nm (use 5 sec mixing mode in the plate reader) was then determined, in order to provide the “blank” reading. For the test, 100 μL / well of 15% (w / v) trichloroacetic acid (TCA) was placed in the plates and incubated between 5 and 30 min at room temperature. The light scattering / absorbance at 405 nm (u...
example 2
Production of GCI-P036 Protease in B. subtilis
[0263]In this Example, experiments conducted to produce GCI-P036 protease in B. subtilis are described. Transformation of expression vector encoding GCI-P036 and variants thereof into B. subtilis cells (ΔaprE, ΔnprE, oppA, ΔspoIIE, degUHy32, ΔamyE::[xylR,pxylA-comK]) was performed as described previously (See e.g., WO 2002 / 014490).
GCI-P036 Protease Production—pAC-GCI-P036ci
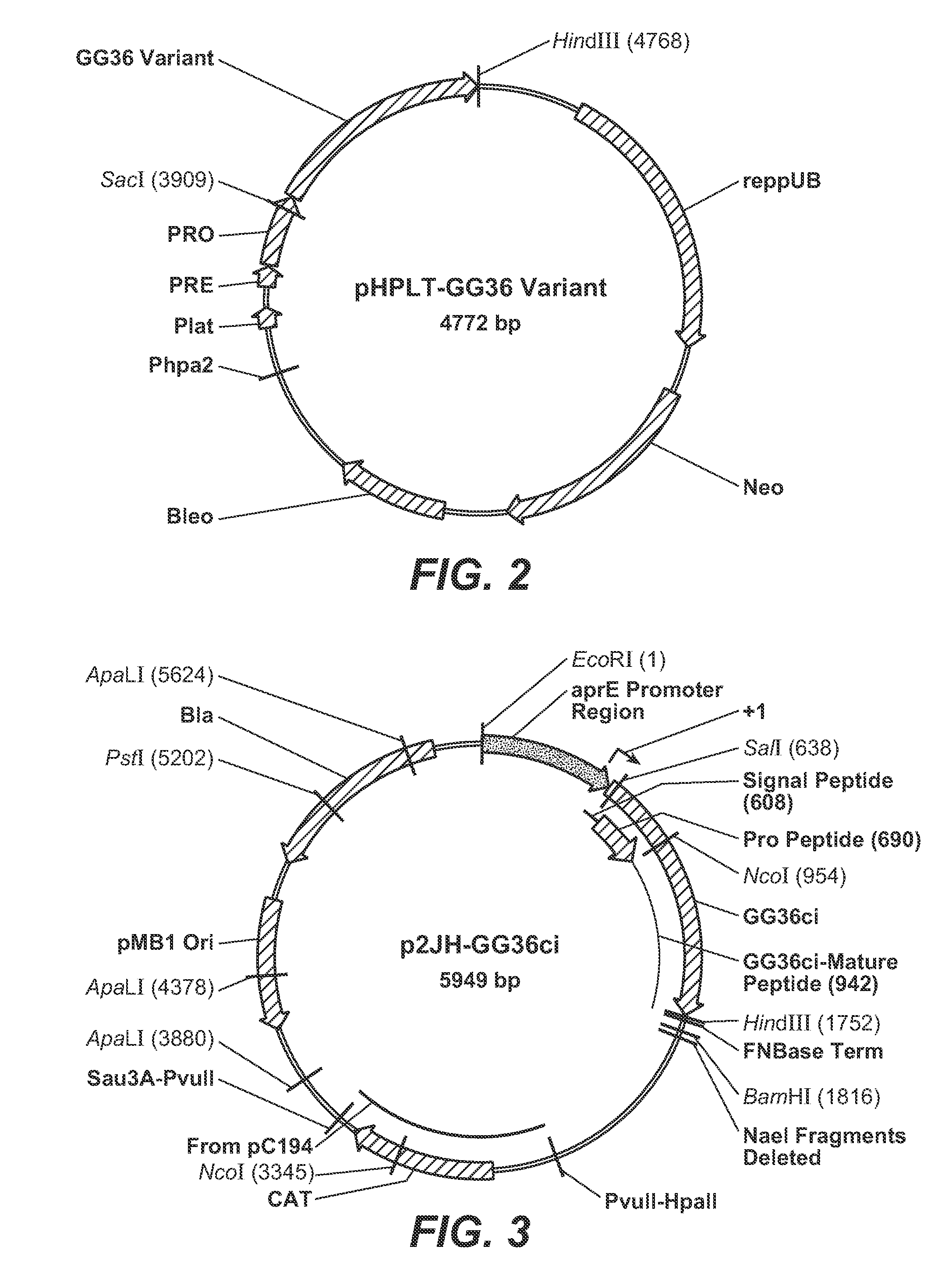
[0264]Exemplary methods to produce GCI-P036 (also referred to herein as “B. lentus subtilisin” and “GG36”) in B. subtilis are provided. The expression plasmid pAC-GG36ci was assembled using the GCI-P036 codon-improved gene fused at the eighth codon of the aprE signal sequence under the control of the consensus aprE promoter and the BPN′ transcriptional terminator. In the sequence provided below, bold and italicized font indicates the consensus aprE promoter, standard font indicates the signal sequence, underlined font indicates the pro sequence, bold font indicates DN...
example 3
Generation of GCI-P036 Variants
[0271]In this Example, methods used to produce the protease variants are described.
Generation of GCI-P036 Site Evaluation Libraries (SELs)
[0272]SEL production was performed by GENEART using a proprietary process (WO 2004 / 059556A3). Methods and devices for optimizing a nucleotide sequence for the purpose of expression of a protein by PCR, and the manufacture of DNA molecules utilized technology owned by or licensed to GENEART (European Patent Nos. 0 200 362 and 0 201 184; and U.S. Pat. Nos. 4,683,195, 4,683,202 and 6,472,184). The construction of GCI-P036 SEL described in this example was performed by GENEART using their methods and technology platform for gene optimization, gene synthesis and library generation and analysis.
[0273]GCI-P036 SELs were produced by GENEART at positions pre-selected by the inventors. The pHPLT-GCI-P036 plasmid DNA (See, FIG. 2) was digested with Sad and HindIII restriction enzymes, releasing a 3.9 kb fragment that was subseq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com