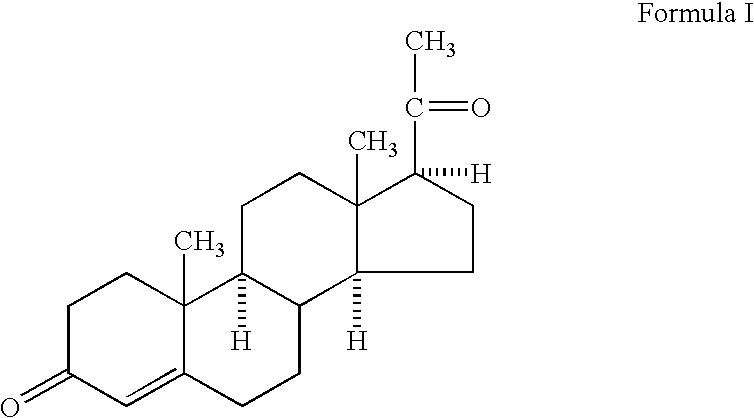
Methods for the treatment of a traumatic central nervous system injury
a central nervous system and traumatic injury technology, applied in the field of traumatic central nervous system injury treatment, can solve the problems of increased patient mortality, morbidity and mortality, and inability to administer progesterone, and achieve the effects of increasing patient mortality, morbidity and mortality, and increasing lesion siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
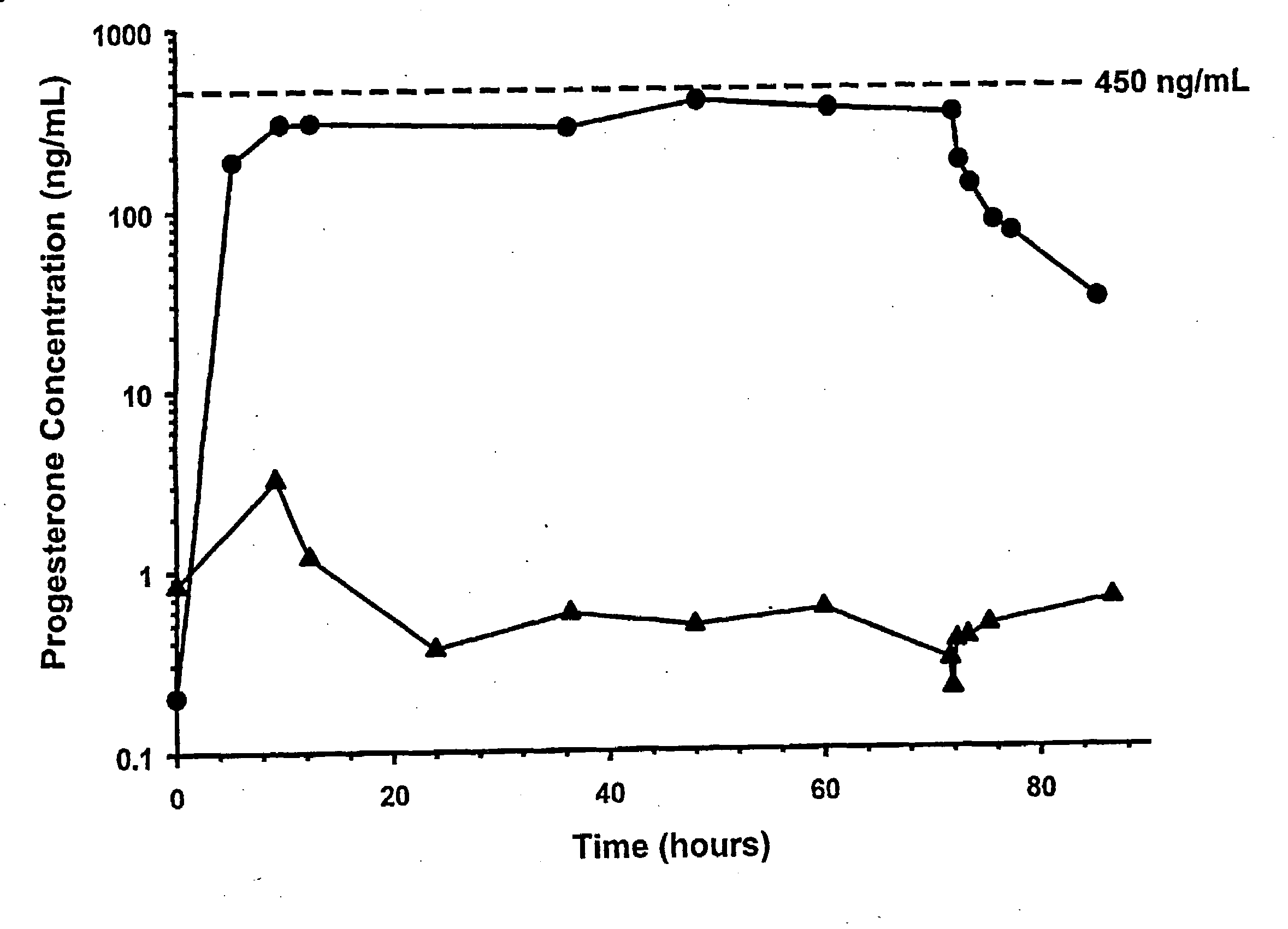
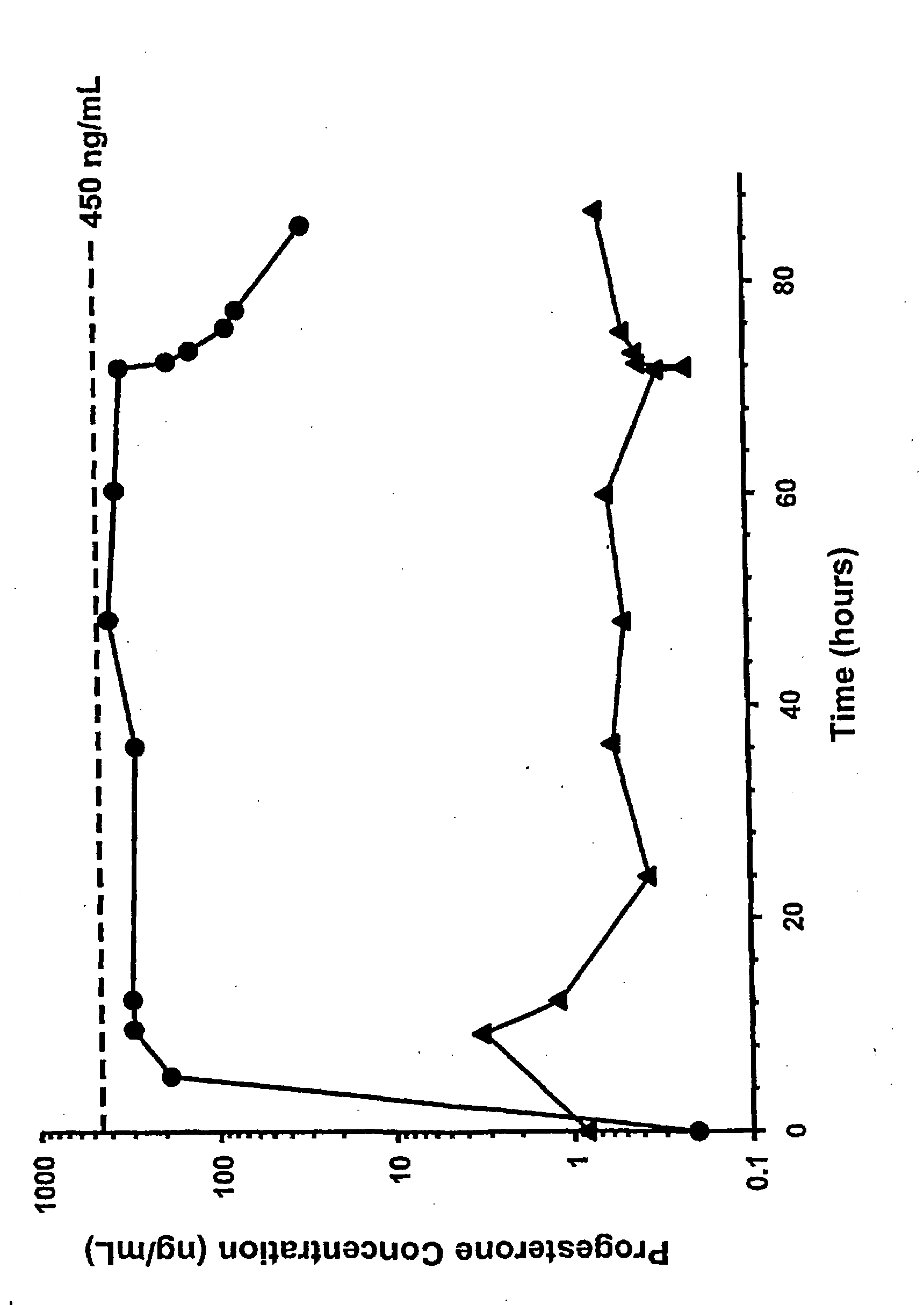
[0076]As a first step in assessing the applicability of progesterone therapy in humans, we examined the effects of acute TBI and extracranial trauma on the pharmacokinetics of PG given by intravenous infusion. Multiple blood samples were obtained from 11 female and 21 male trauma patients receiving PG and 1 female and 3 male patients receiving placebo infusions for 72 h. Values for CSS, CL, t1 / 2 and Vd were obtained using AUC(0-72) and post-infusion blood samples. CSS values were 337±135 ng / mL, which were significantly lower than the target concentration of 45±100 ng / mL. The lower CSS is attributed to the CL, which was higher than anticipated. In addition, t1 / 2 was longer and Vd was higher than anticipated. There were no significant gender differences in any of these parameters. These changes are consistent with the hyperkinetic changes associated with critical injury. Our results demonstrate that stable PG concentrations can be rapidly achieved following TBI.
Methods
Patient Selecti...
example 2
[0095]A pilot phase II, randomized, double-blind, controlled trial of progesterone for the treatment of a traumatic brain injury was preformed. The administration protocol was carried out was described above in Example 1.
[0096]To determine if a therapeutic response was achieved, the following endpoints were monitored:
[0097]ICP reduction determined by calculating “therapeutic intensity level” (ICP-TIL);
[0098]duration of coma (injury to awaking);
[0099]mortality one-month post injury;
[0100]neurological outcome 1 month and 1 year post-injury, as determined by Glasgow outcome scale (GOS), Disability rating scale (DRS) and Galveston orientation and amnesia test (GOAT).
[0101]The preliminary evaluations are as follows. One hundred patients having moderate to severe TBI were enrolled in the study, which had a randomized block design 4:1 enrollment. Three days IV administration of progesterone [450+ / −mmol / L] in both males and females. The administration protocol and pharmaceutical composition...
example 3
[0104]We conducted a clinical trial to assess the safety of progesterone as a treatment for acute TBI. This phase II, randomized, double blind, placebo-controlled clinical trial was conducted at an urban, level I trauma center. 100 adults presenting within 11 hours of a blunt TBI with a Glasgow Coma Scale score of 4-12 were enrolled with proxy consent. Subjects were randomized on a 4:1 basis to progesterone versus placebo. Blinded observers closely monitored patients for the occurrence of adverse events, and initial functional outcomes were assessed 30 days post-injury. The primary safety outcome was difference in adverse event rates, including mortality. The primary measure of activity was dichotomized Glasgow outcome scale extended (GOSE) 30 days post injury. Seventy-seven patients received progesterone; 23 received placebo. The groups had very similar demographic and clinical characteristics. With the exception of mortality, the rate of adverse events was similar in both groups. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com