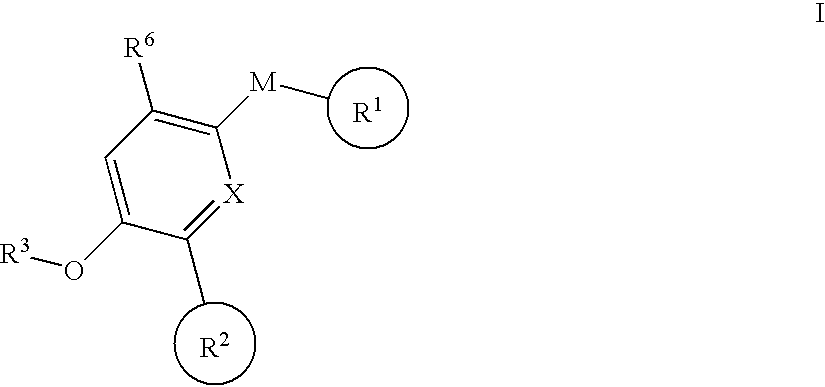
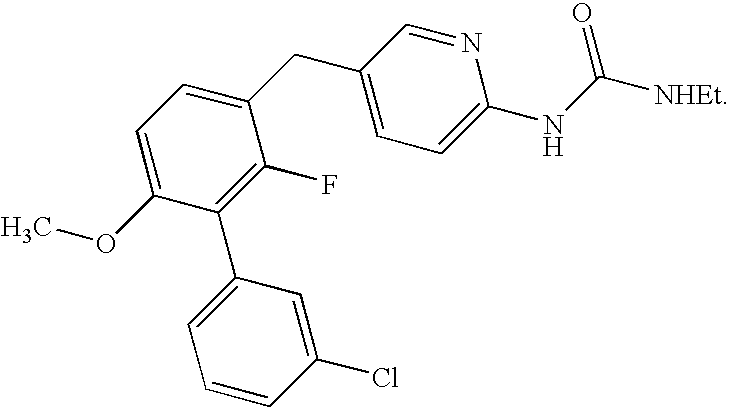
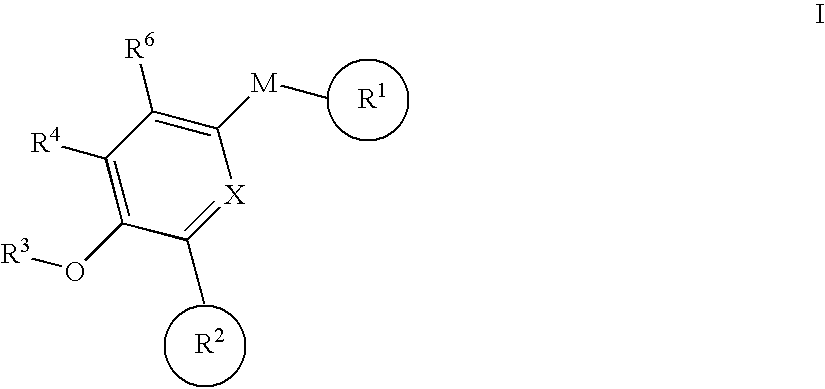
Biaryl pde4 inhibitors for treating inflammatory, cardiovascular and CNS disorders
a technology of pde4 inhibitors and biaryl pde4, which is applied in the direction of respiratory disorders, cardiovascular disorders, drug compositions, etc., can solve the problems of low therapeutic ratio, severe limits on the dose that can be given, and no drug has yet reached the market, so as to improve cognition and treat or prevent a disease.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of P-065
[0111]
[0112]Synthesis of 2-bromo-6-methyl-pyridin-3-ol (I-2, X═Br, Y═CH3): To 6-methyl-pyridin-3-ol (I-1, Y═CH3, 5.0 g, 45.82 mmol) in pyridine (15 mL) was added bromine (3.66 g, 22.91 mmol). The reaction was stirred at room temperature under N2 for 20 h. The crude reaction mixture was poured on to crushed ice-water (300 mL), stirred for 3 h. The mixture was extracted with ethyl acetate (5×100 mL) and the combined organic extracts were washed with brine, dried over Na2SO4, filtered, and concentrated to afford 6.3 g (73%) of 2-bromo-6-methyl-pyridin-3-ol (I-2, X═Br, Y═CH3) as light yellow solid.
[0113]Synthesis of 2-bromo-3-methoxy-6-methyl-pyridine (I-3, X═Br, Y═R1═CH3): To the 2-bromo-6-methyl-pyridin-3-ol (I-2) 6.0 g, 31.91 mmol) and K2CO3 (8.82 g, 63.82 mmol) in acetone (100 mL) was added MeI (6.79 g, 479.87 mmol). The reaction was stirred at 45° C. under N2 for 20 h. The reaction was cooled to room temperature, filtered and concentrated. The residue was purifi...
example 2
Preparation of P-176
[0118]
[0119]Synthesis of 2-bromo-3-nitro-phenol (I-8, X═Br): (Prepared by a modification of reported procedure, J. Org. Chem. 1988, 53, pp 1170-1176). To 2-amino-3-nitro-phenol (24.9 mmol, 1.0 eq.) in 24 mL of water and 12 mL of 1,4-dioxane at reflux, was added 13 mL of HBr (48% aq.) over 10 minutes. The resulting solution was refluxed for an additional 15 minutes, and cooled 0-5° C. A solution of sodium nitrite (24.4 mmol, 0.98 eq.) in 20 mL of water was added over 10 minutes, and stirred for 15 minutes. The reaction mixture was then heated to 60° C. for 15 minutes, and allowed to cool naturally to room temperature, and stirred for 16 hours. The reaction mixture was then extracted with two portions of diethyl ether, and the combined ethereal layers washed with brine, dried over magnesium sulfate, filtered through a layer of celite and concentrated. The residue was diluted with dichloromethane (with ˜0.1% MeOH), and purified via silica gel plug filtration with di...
example 3
Preparation of P-404
[0125]
[0126]Synthesis of 3′-chloro-6-fluoro-2-methoxy-biphenyl (I-16, R1═CH3, R2═Cl, Y═F): To 2-bromo-3-fluoroanisole (1.0 g, 4.88 mmol), 3-chlorophenylboronic acid (0.91 g, 5.88 mmol), PPh3 (0.64 g, 2.44 mmol), K2CO3 (0.27 g, 1.95 mmol) and Pd(OAc)2 (0.13 g, 0.58 mmol) was added dioxane (8 mL), and EtOH—H2O (1:1, 4 mL). Ar gas was bubbled through the stirred reaction for 5 min. The reaction was heated at 180° C. using microwave oven (Biotage Intiator II) for 20 min. The reaction was cooled to room temperature, combined with another 0.5 g scale run, concentrated. The residue was purified by silica gel column chromatography using 1:1 dichloromethane-hexanes to afford 1.33 g (77%) 3′-chloro-6-fluoro-2-methoxy-biphenyl (I-16, R1═CH3, R2═Cl, Y═F) as a viscous liquid.
[0127]Synthesis of N-[4-(3′-chloro-2-fluoro-6-methoxy-biphenyl-3-carbonyl)-phenyl]-acetamide (I-19, R1═CH3, R2═Cl, Y═F, R3=4-acetylaminophenyl): To a stirred suspension of 4-acetylaminobenzoic acid (Aldri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com