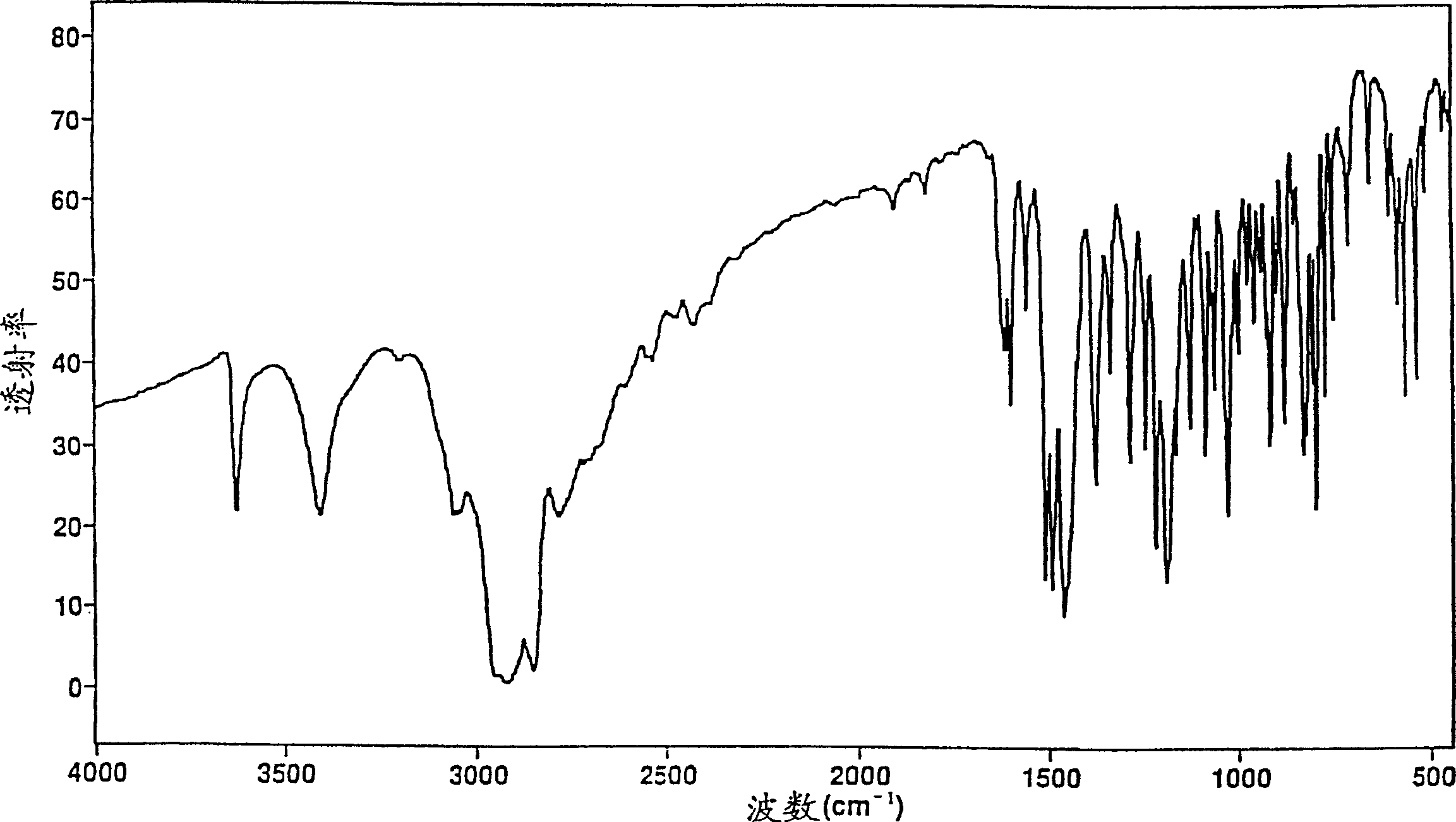
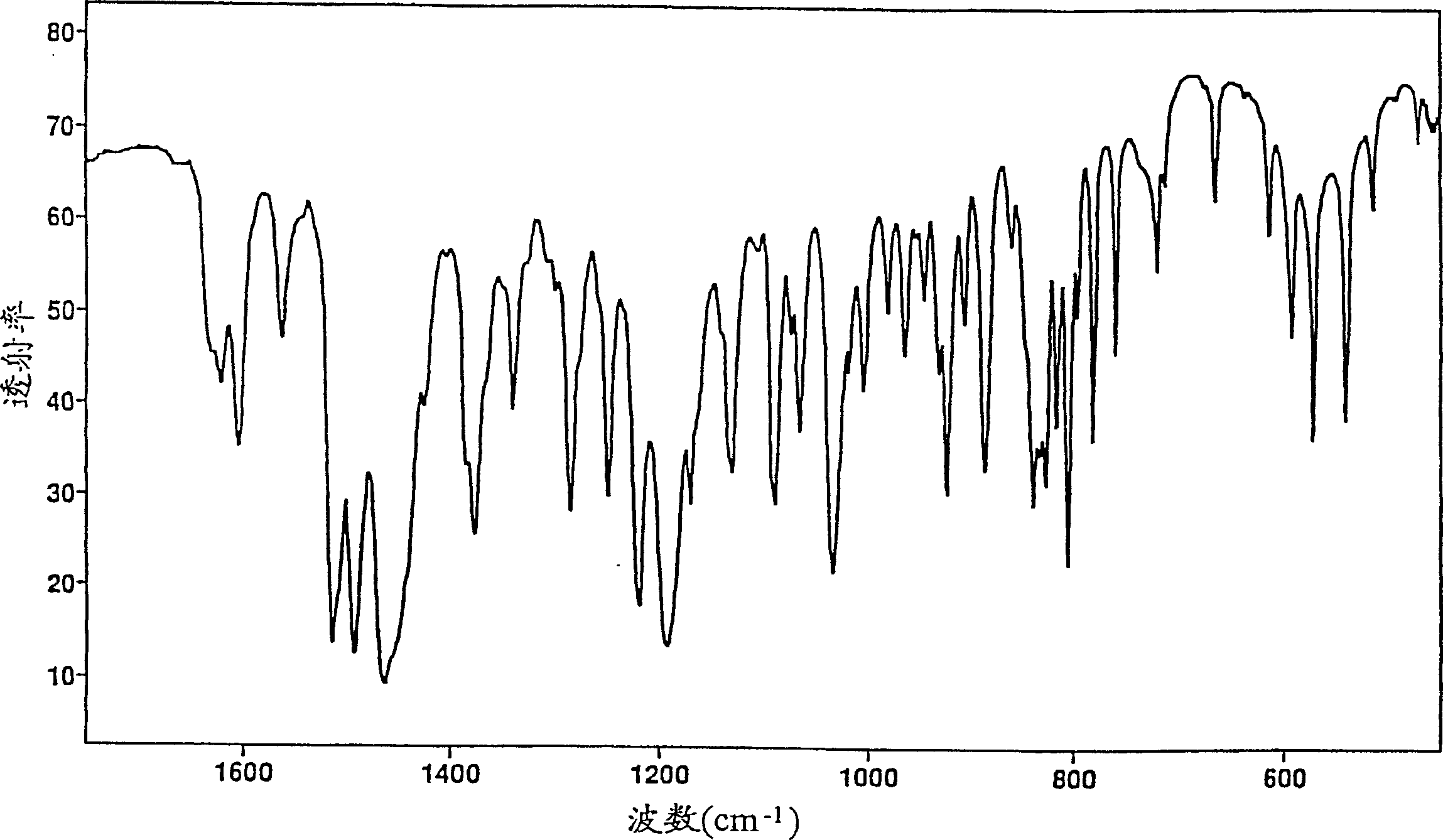
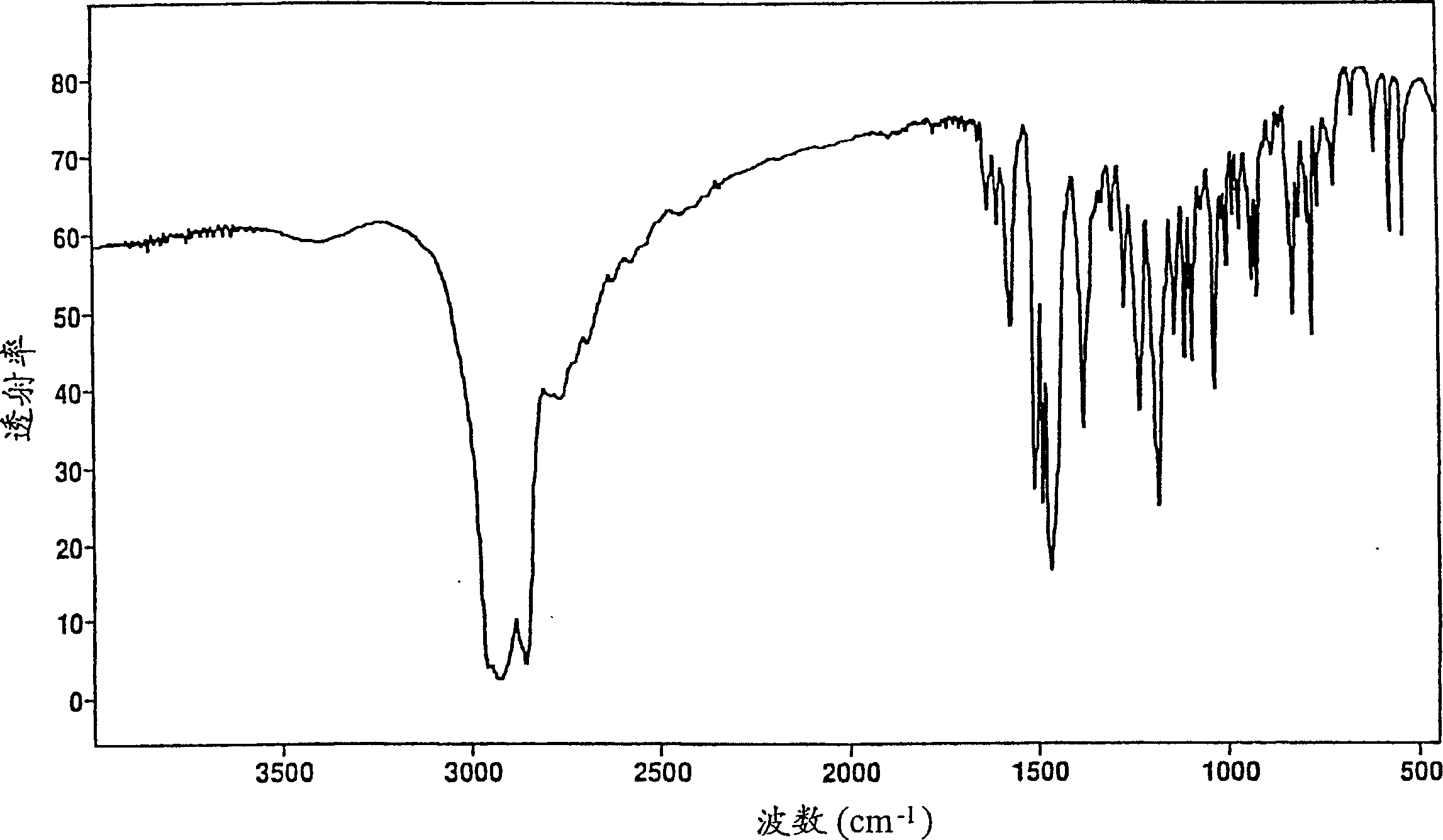
New compound
A technology for paroxetine hydrochloride and hydrate, which is applied to the new paroxetine hydrochloride anhydrate and the fields of its preparation and use, can solve the problem that the paroxetine hydrochloride anhydrate cannot be produced, and the anhydrate is not clear. teaching, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Crystalline paroxetine hydrochloride anhydrate substantially free of bound propan-2-ol (Form A)
[0111] i) Paroxetine hydrochloride propan-2-ol solvate
[0112] Paroxetine hydrochloride hemihydrate (150 g) was stirred with propan-2-ol (1000 ml) and toluene (300 ml) in a round bottom flask and heated to boiling. The solvent was distilled off. The total volume was kept constant by adding fresh propan-2-ol until the boiling point reached about 82°C, indicating that the water had been removed.
[0113] While simultaneously crystallizing, the mixture was cooled to about 50°C. The contents of the flask quickly turned to a thick paste, which was diluted with propan-2-ol (ca. 500 mL) and stirred vigorously. The resulting suspension was cooled to about 30°C and vacuum filtered, taking care to prevent absorption of atmospheric moisture. The solvent-containing filter cake was dried over phosphorus pentoxide under high vacuum.
[0114] 151 g of the solvated paroxetine hydroch...
Embodiment 2
[0127] Paroxetine hydrochloride propan-2-ol solvate
[0128] Paroxetine free base (42.09 g) was dissolved in propan-2-ol (Fisons SLR grade, 210 ml). Hydrogen chloride gas was bubbled into the cooled flask containing propan-2-ol (157 g) until 20.8 g of HCl gas was absorbed. 39 g of this solution (containing approximately 4.6 g HCl) was quickly added to the paroxetine solution and the mixture was stirred rapidly. Crystallization started after about 1 minute and the mixture quickly became an unstirable paste which was left for an hour. The product was collected by filtration, washed with propan-2-ol (50 ml), and vacuum-dried at room temperature in a desiccator containing phosphorus pentoxide to constant weight. A sample was analyzed by NMR spectroscopy and found to contain about 6% by weight propan-2-ol. Some samples were further dried to constant weight in a vacuum oven at 50°C, which took 4 days. NMR spectroscopy indicated that the sample contained about 2% by weight propan...
Embodiment 3
[0130] Paroxetine hydrochloride propan-2-ol solvate
[0131] Paroxetine free base (52.37g) was dissolved in anhydrous propan-2-ol (250ml) and a solution of HCl gas in propan-2-ol (50ml solution containing about 5.8g HCl) was added rapidly with rapid stirring. After 30 seconds crystallization occurred and the mixture was stirred at room temperature for 30 minutes to allow complete crystallization. The product was isolated by suction filtration, washed with 25 ml of anhydrous propan-2-ol, and dried in a desiccator containing phosphorus pentoxide under vacuum at room temperature.
[0132] A sample was analyzed by NMR after 3 days and found to contain 10.5% propan-2-ol. The rest of the material was re-dried in a desiccator with fresh phosphorus pentoxide for 3 days to constant weight. NMR indicated that the product contained 4.7% (w / w) propan-2-ol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com