Application of itaconic acid in preparation of medicine for treating or relieving allergic airway inflammation diseases
A technology of airway inflammation and itaconic acid, applied in the direction of allergic diseases, drug combinations, respiratory diseases, etc., to achieve significant effects and broaden the effect of drug types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
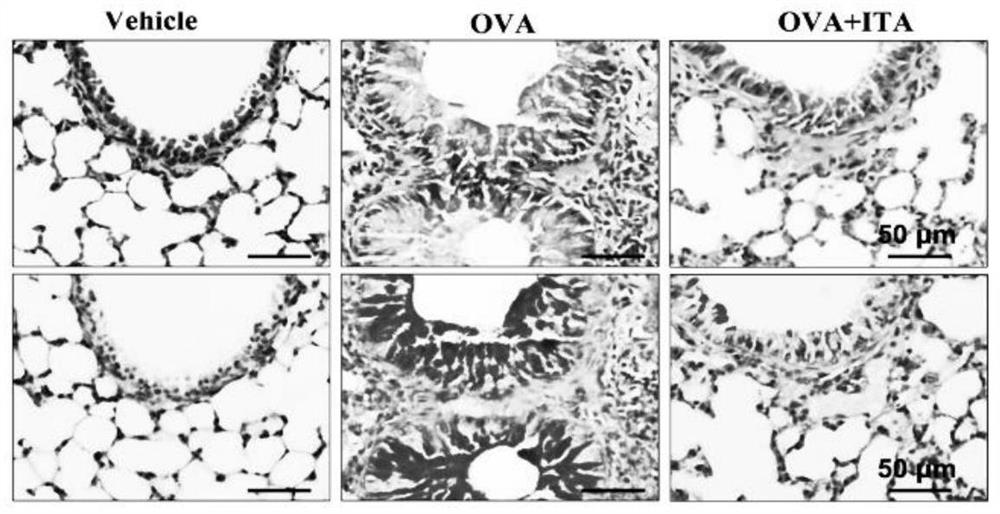
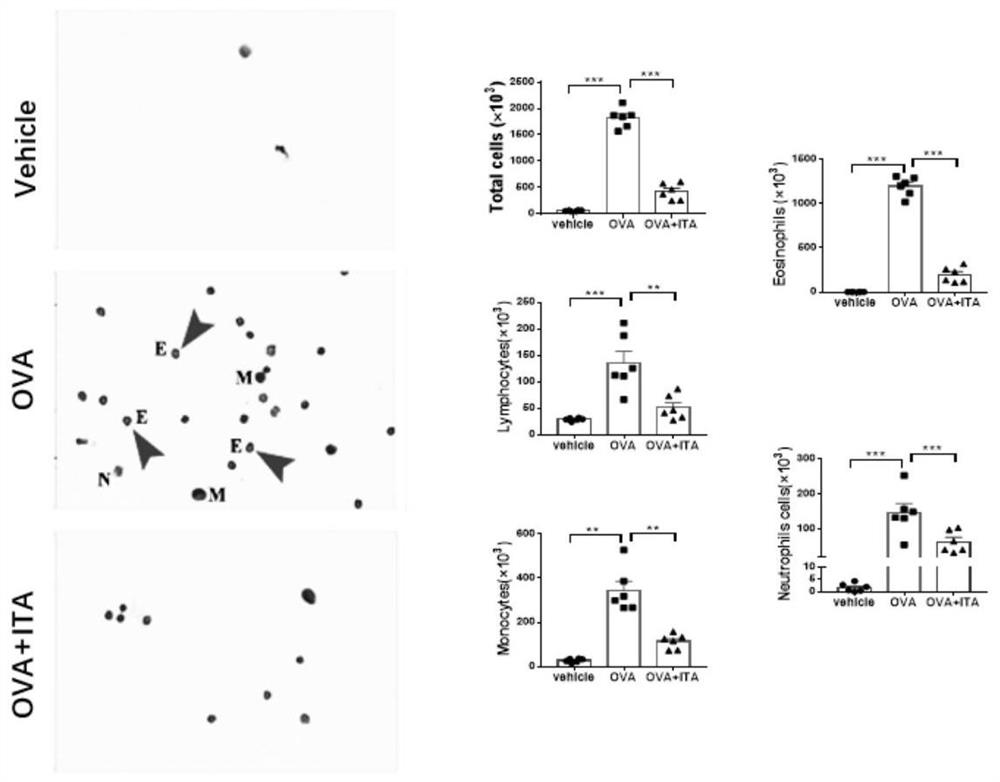
[0031] Example 1: In vivo experiments verify that itaconic acid intervention effectively inhibits OVA-induced allergic airway inflammation;
[0032] (1) Experimental animals:
[0033] Eighteen SPF (specific pathogen free) female C57BL / J mice aged 5 to 6 weeks (14 to 16 g) were purchased from the Experimental Animal Center of Anhui Province, and randomly divided into control group (Vehicle group) and model group (OVA group) and itaconic acid intervention group (OVA+ITA group). Experiments were repeated three times.
[0034] (2) Allergic airway inflammation model:
[0035] After 3 days of adaptation, the model group and the itaconic acid intervention group were injected intraperitoneally with 0.5ml ovalbumin (Ovalbumin, OVA) suspension (containing 1mg aluminum potassium sulfate and 10ug ovalbumin) on the 0th day and the 7th day, and the control group Inject an equal volume of normal saline solution (containing 1mg aluminum potassium sulfate). From the 14th day, the model gro...
Embodiment 2
[0048] Embodiment 2: in vitro experiment
[0049] (1) The mouse macrophage cell line Raw264.7 was purchased from the Cell Bank of the Chinese Academy of Sciences, and the cells were planted in a 96-well culture plate at a density of 2.5×10^4 / well, and were divided into three groups, the control group, Staphylococcus aureus stimulated group, itaconic acid intervention group. Raw264.7 cells were stimulated with Staphylococcus aureus (S.aureus, strain NCTC8325), and the intervention group was treated with itaconic acid (100uM).
[0050] (2) Mitochondrial ROS and mitochondrial membrane potential detection, MitoSox Red (5 μM) staining was used to detect mitochondrial ROS in Raw264.7 cells, and MitoTracker Red CMXRos (1 μM) staining was used to detect mitochondrial membrane potential.
[0051] (3) XFe96 cell energy metabolism analyzer (Agilent, Seahorse Bioscience) was used to determine the effect of itaconic acid on mitochondrial respiratory function in Raw264.7 cells stimulated b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com