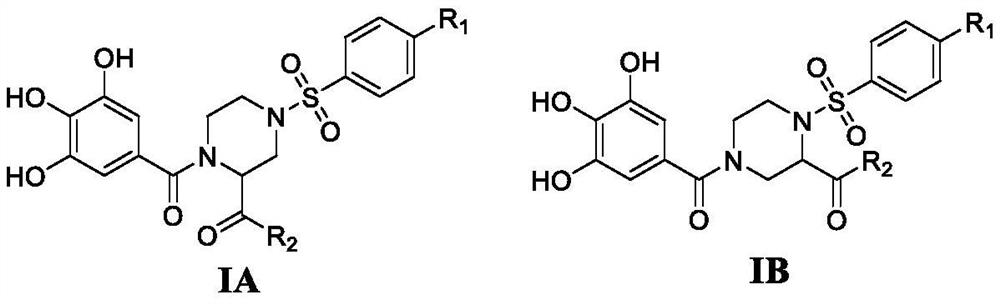
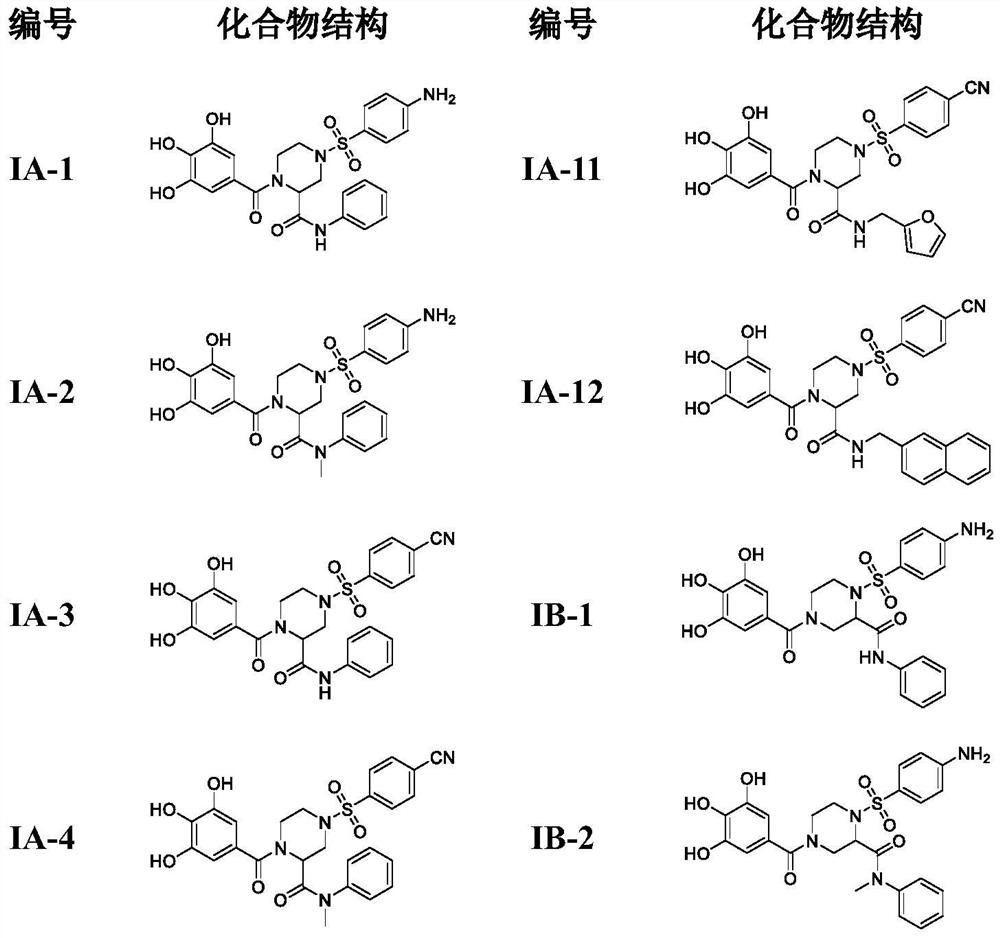
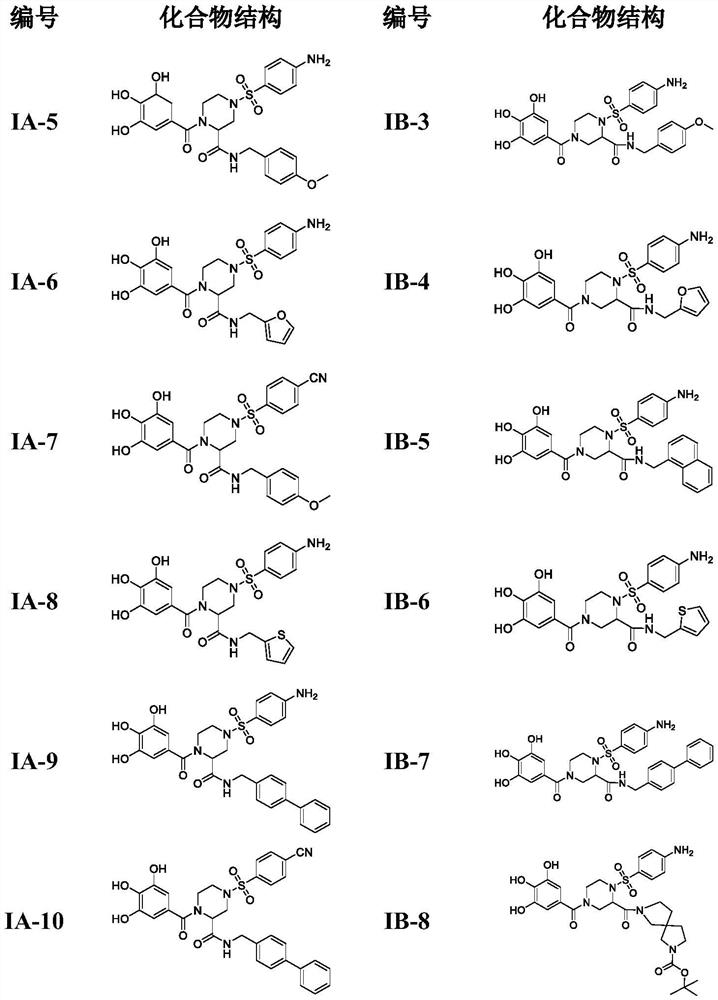
4-phenylsulfonyl-1-trihydroxybenzoylpiperazine-2-carboxamide derivative and its preparation method and application
A kind of technology of trihydroxybenzoylpiperazine and phenylsulfonyl, applied in the field of derivatives and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Intermediate 1-(tert-butyl)3-methyl 4-(3,4,5-tri(benzyloxy)benzoyl)piperazine-1,3-dicarboxylate (IA- b) Preparation
[0043] Weigh 3,4,5-tribenzyloxybenzoic acid (2.0180g, 4.52mmol) into a 250mL flask, add 50mL N,N-dimethylformamide to dissolve, add 1-(3- Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.9322 g, 4.89 mmol), 1-hydroxybenzotriazole (0.6425 g, 4.97 mmol), triethylamine (1.25 mL, 10.06 mmol) ), stirred for 15 min, removed the ice bath, and stirred at room temperature for 1 h. Finally, 1-Boc-3-methylpiperazine-1,3-dicarboxylate or 1-Boc-2-methylpiperazine-1,2-dicarboxylate (1.0925 g, 5.51 mmol) was added to the flask ) and reacted overnight. After the reaction was completed, 150 mL of water was added to the reaction solution, resulting in white turbidity. After mixing, it was transferred to a separatory funnel, extracted with ethyl acetate (3×50 mL), and the organic phases were combined, followed by 1N hydrochloric acid solution and sat...
Embodiment 2
[0044] Example 2: Preparation of intermediate 1-(3,4,5-tris(benzyloxy)benzoyl)piperazine-2-carboxylic acid methyl ester (IA-c)
[0045] IA-b (1.2305 g, 2.51 mmol) was weighed and dissolved in 5 mL of dichloromethane, then trifluoroacetic acid (2.12 mL, 30 mmol) was slowly added thereto, and the mixture was stirred at room temperature for 6 h. TLC detected the completion of the reaction, added 10 mL of water to the reaction solution, adjusted the pH to 9 with saturated sodium bicarbonate solution, extracted three times with dichloromethane, washed 2-3 times with saturated sodium chloride solution, combined the organic layers, anhydrous sodium sulfate dry. Then through flash column chromatography, recrystallization from ethyl acetate-petroleum ether system gave a pale yellow solid, yield: 81%, mp: 156-158°C. 1 H NMR (400MHz, DMSO-d 6 )δ7.42(t,J=6.7Hz,6H),7.35(dd,J=7.1,1.1Hz,3H),7.39–7.25(m,6H),6.95(s,2H),5.16(s,6H ), 4.78(t, J=5.2Hz, 1H), 4.03(m, 6H), 3.67(s, 3H), 1.08(s, 1H,...
Embodiment 3
[0046] Example 3: Intermediate 4-((4-nitrophenyl)sulfonyl)-1-(3,4,5-tris(benzyloxy)benzoyl)piperazine-2-carboxylic acid methyl ester ( Preparation of IA-d-1)
[0047] Weigh the intermediate IA-c (1.5012g, 0.18mmol) and add it to a 50mL flask, add 20mL of dichloromethane to dissolve, and add triethylamine (0.75mL, 0.36mmol) and p-nitrogen to the reaction flask in turn under ice bath conditions benzenesulfonyl chloride (0.9212 g, 0.27 mmol), removed the ice bath after 30 min, and stirred at room temperature for 12 h. TLC detected the completion of the reaction, concentrated the reaction solution, extracted three times with ethyl acetate, combined the organic phases, washed with 1N hydrochloric acid solution and saturated sodium bicarbonate solution for 2-3 times in turn, and finally washed with saturated sodium chloride solution for 1-2 times Second-rate. The organic phase was transferred to a 250 mL conical flask, an appropriate amount of anhydrous sodium sulfate was added, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com