Application of tempol in the preparation of drugs for the treatment of polycystic ovary syndrome
A technology for polycystic ovary syndrome, applied in the field of application in the preparation of drugs for the treatment of polycystic ovary syndrome, to achieve broad application prospects, improve PCOS, alleviate the effect of abundance and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The construction of embodiment 1 animal model
[0075] Three-week-old SD rats were divided into three groups: control group (Oil+PBS group), PCOS model group (DHEA+PBS group), Tempol treatment group (DHEA+Tempol group). There were 6-11 rats in each group, and the construction method of the rat model in the control group was as follows: 200 μL of sesame oil was injected subcutaneously, and after 21 days of continuous injection, PBS was injected intraperitoneally for 12 consecutive days; Hydroepiandrosterone (DHEA) (Solarbio, D8950) 6mg / 100g, 200 μ L, after continuous injection for 21 days, intraperitoneal injection of PBS for 12 consecutive days; the construction method of the Tempol treatment group rat model is as follows: subcutaneous injection of DHEA ) 6mg / 100g, 200μL, after continuous injection for 21 days, Tempol (30mg / kg) (Selleck Chemicals LLC#S2910, Houston, TX, USA) was injected intraperitoneally for 12 consecutive days. Weighing was performed weekly during th...
Embodiment 2
[0077] Example 2 The observation of rat estrous cycle, the observation of ovarian histomorphology and the measurement and analysis of serum hormones 1, the observation of rat estrous cycle
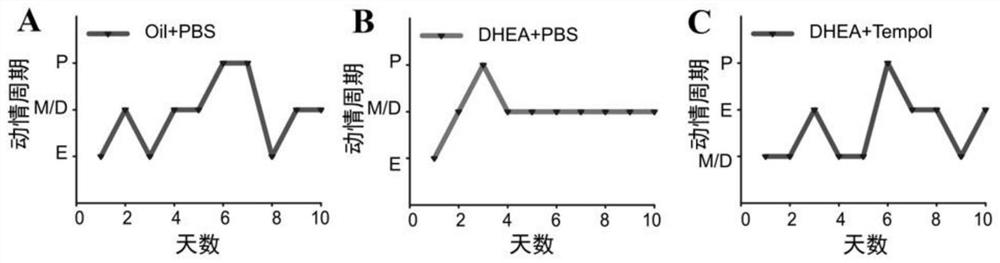
[0078] Rats are animals with multiple estrus throughout the year and have a relatively stable sexual cycle, which is divided into: preestrus (P), estrus (E), postestrus (M) and diestrus (D). The vaginal mucosa of each period is Typical changes occur, which stage can be judged according to the cytological characteristics of the vaginal smear. In this embodiment, 3 rats in the control group (Oil+PBS group), PCOS model group (DHEA+PBS group), and Tempol treatment group (DHEA+Tempol group) three groups of experimental animals were taken from the vagina at the same time every afternoon. The cell smears were stained with Wright's staining solution (LEAGENE, 1029A20) for 10 consecutive days to observe the cell morphology and record and count the experimental results respectively.
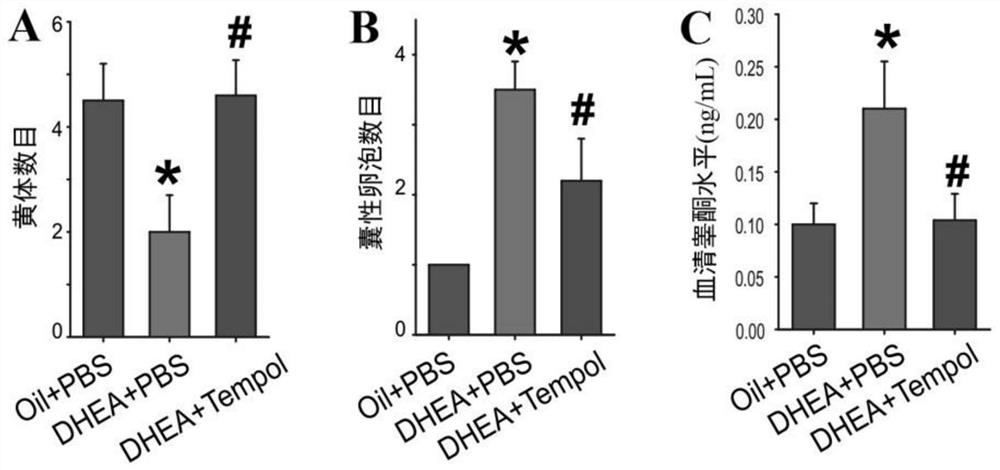
[0079] 2. HE ...
Embodiment 3
[0086] Example 3 Statistical analysis on biomarker level
[0087] 1. 16S rRNA gene sequencing
[0088] Total bacterial DNA was extracted from feces using a DNA isolation kit (MO BIO Laboratories, Carlsbad, CA, USA). The quality and quantity of DNA were evaluated by the ratio of 260nm / 280nm and 260nm / 230nm. DNA was then stored at -80°C until further processing. The V3-V4 region of the bacterial 16S rRNA gene was amplified with common primers (SEQ ID NO.1-SEQ ID NO.2) in combination with the adapter sequence and the barcode sequence. Thermal cycling conditions were: initial denaturation at 95°C for 5 min, followed by 15 cycles of denaturation at 95°C for 1 min, 50°C for 1 min and 72°C for 1 min, and finally extension at 72°C for 7 min. The first step PCR product is purified by VAHTSTM DNA cleaning beads. The second round of PCR was performed in a 40 μL reaction consisting of 20 μL 2× Phμsion HF-MM, 8 μL ddH 2 O. 10 μM of each primer and 10 μL of the PCR product from the fir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com