Electrochemical device, method for electrochemically decomposing urea to synthesize hydrogen peroxide, and application
A hydrogen peroxide and electrochemical technology, applied in the field of chemistry, can solve the problem of high input cost, and achieve the effect of solving high input cost, reducing production cost, and low modularization of equipment and equipment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
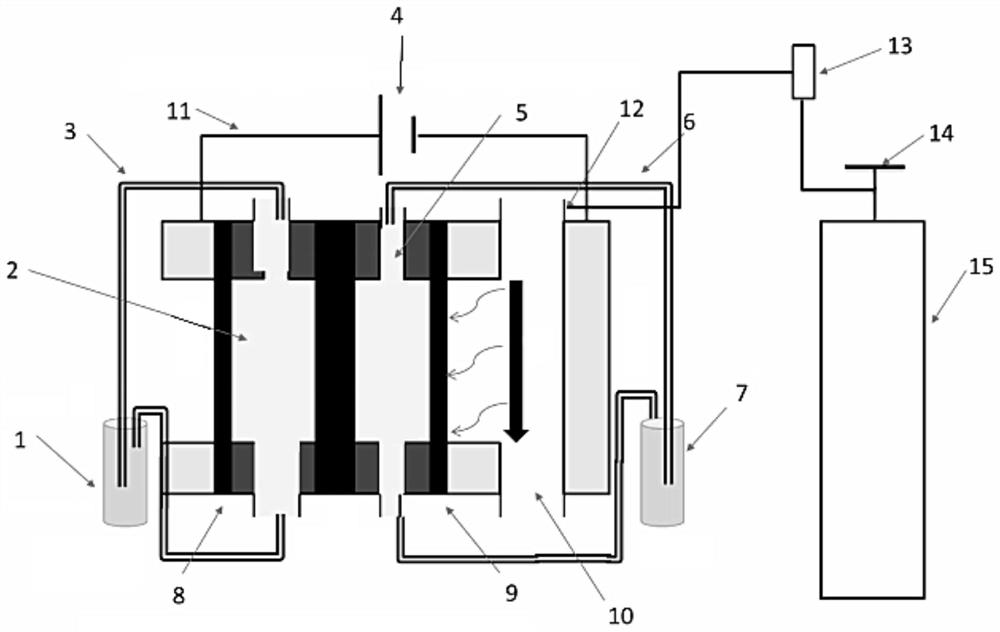
[0067] In the electrolytic cell with the anode electrode 8 and the cathode electrode 9 separated by the proton exchange membrane, the anode electrode 8 uses a platinum sheet as the working electrode, and the catalyst uses a high-efficiency UOR catalyst; the cathode electrode 9 uses a copper sheet as the electrode, and the catalyst uses CMK-3 , the reference electrode is a silver / silver chloride electrode, a solution containing KOH (concentration 1mol / L) and urea (concentration 0.33mol / L) is circulated in the anode reaction chamber 2, and KOH (concentration 1mol / L) is circulated in the cathode reaction chamber 5. L) solution, and feed oxygen into the gas phase chamber 10, under the condition of continuous feeding, the constant potential synchronously decomposes urea and synthesizes hydrogen peroxide, and the potential is controlled at 1.2v (relative to the silver / silver chloride electrode) (without IR Correction).
[0068] The preparation process provided in Example 1 of the pr...
Embodiment 2
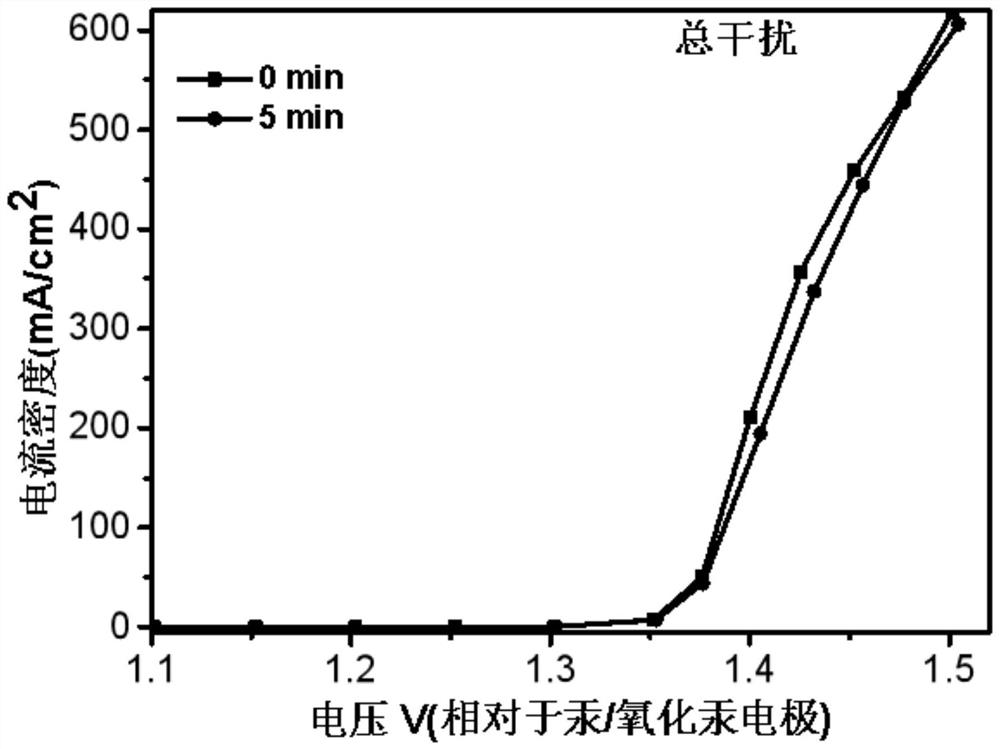
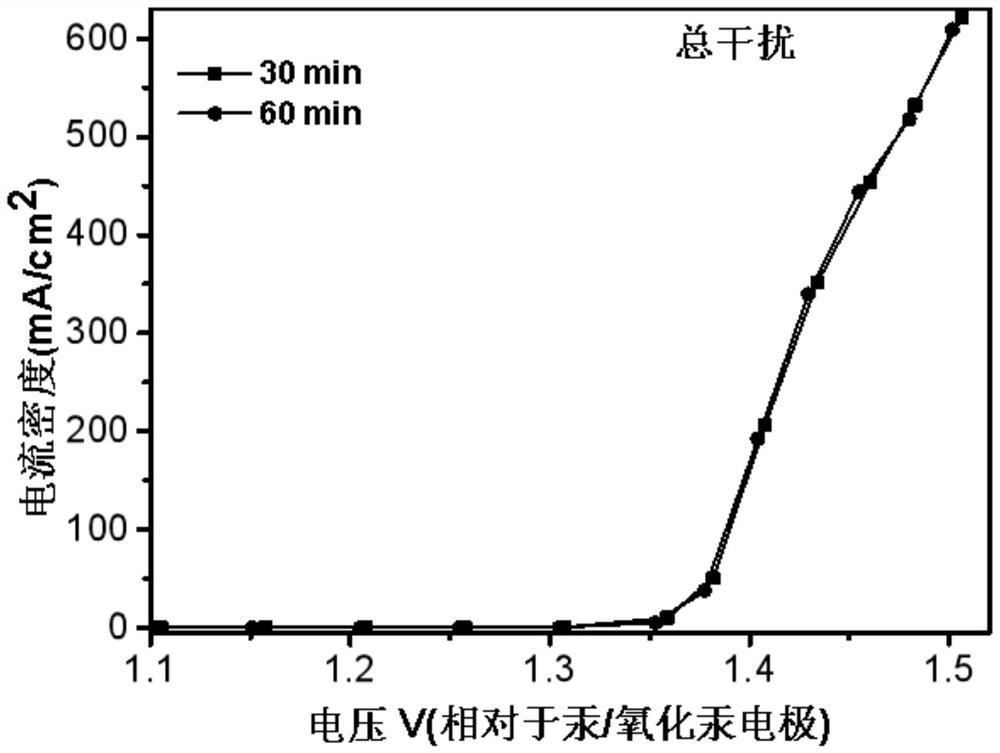
[0071] In an embodiment of the present invention, an electrochemical device, in an electrolytic cell separated by a proton exchange membrane into an anode electrode 8 and a cathode electrode 9, also uses a platinum sheet as a working electrode, the anode is a UOR catalyst, and the reference electrode is Mercury / mercuric oxide electrode, the negative electrode is electrode with copper sheet, and catalyst is CMK-3, and anolyte is the simulation human urine, and catholyte is KOH (concentration 1mol / L), and feeds oxygen to gas phase chamber 10, in In the case of continuous access, carry out LSV (Linear Sweep Voltammetry) test, the test time is 0min, 5min, 30min, 60min to measure LSV at each time, and the results are as follows: figure 2 and image 3 LSV plots under simulated urine conditions are shown. in, figure 2 It is the LSV curve graph under simulated urine conditions with the test time being 0min and 5min respectively, image 3 It is the LSV curve graph under simulated ...
Embodiment 3
[0074] The three-electrode system of the electrochemical device provided in embodiment 3 is carried out stability test, with platinum sheet as counter electrode, anode adopts platinum sheet as electrode, anode is UOR catalyst, reference electrode adopts mercury / mercuric oxide electrode, cathode uses The copper sheet is an electrode, the catalyst is CMK-3, the anolyte is simulated human urine, the catholyte is KOH (concentration 1mol / L), and oxygen is passed into the gas phase chamber 10, and the current-time test is carried out to obtain the following: Figure 4 The current-time graph is shown. The results show that the stability preference of the electrolysis system can well meet the actual industrial needs.
[0075] The above-mentioned embodiments of the present invention provide an electrochemical device, including an electrolytic reaction device and a gas delivery device. The electrolytic reaction device is used to decompose urea at the anode under the action of a catalyst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com