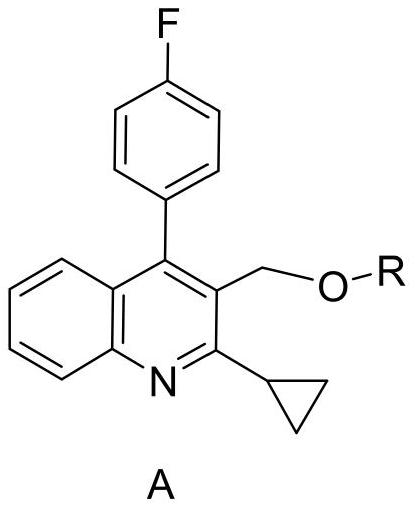
A kind of new preparation method of pitavastatin calcium intermediate
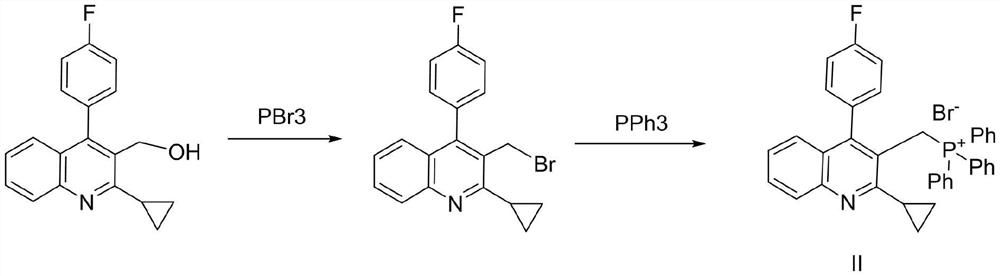
A compound, triphenylphosphine hydrobromide technology, applied in the field of pharmaceutical synthesis, can solve the problems of poor stability of phosphorus-containing wastewater intermediates, loss of advantages, etc., to avoid the generation of unstable brominated intermediates, reduce the content of brominated intermediates, etc. Phosphorus wastewater and phosphorus-containing waste residue, suitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
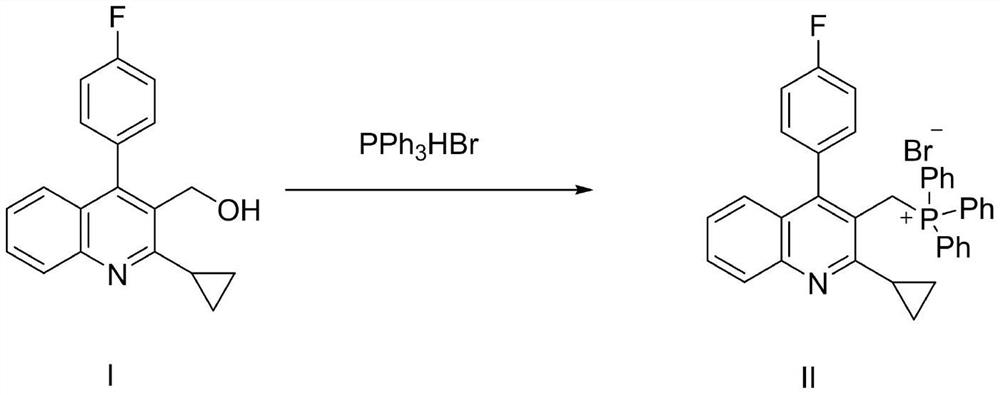
[0025] Embodiment 1: the preparation of compound II
[0026] Add 2.9g of 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol (compound I), 3.4g of triphenylphosphine hydrobromide, and 30ml of toluene into a 100ml three-necked flask, and heat to reflux Stirring, cooling and crystallization after completion of the reaction, and drying to obtain a white solid. That is [[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]methyl]triphenylphosphine bromide (compound II). Yield: 80%; Purity: 99.0%;
[0027] 1H NMR (500MHz, CD3OD): δ=0.64(br s, 2H), 1.05(br s, 2H), 2.04(m, 1H), 5.25(d, J=11.5Hz, 2H), 6.83(br s, 2H), 7.15(d, J=8.4Hz, 1H), 7.18–7.33(m, 8H), 7.38(m, 1H), 7.64(m, 6H), 7.71(m, 1H), 7.89(m, 3H ),7.94(d,J=8.4Hz,1H).
Embodiment 2
[0028] Embodiment 2: the preparation of compound II
[0029] 2.9g of 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol, 3.4g of triphenylphosphine hydrobromide, and 30ml of acetonitrile were added to a 100ml three-necked flask, heated to reflux and stirred, and after the reaction was complete Concentration to dryness afforded a white solid. That is [[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]methyl]triphenylphosphine bromide (compound II). Yield: 96.5%; Purity: 98.0%; Embodiment 3~7: the preparation of compound II
[0030] According to the method shown in Example 1, Compound II was prepared by adjusting the ratio between Compound I and triphenylphosphine hydrobromide and the type of solvent. The results are shown in Table 1.
[0031] Preparation of Table 1 Compound II
[0032]
[0033] As can be seen from Table 1, considering the comprehensive factors of the yield and purity of compound II, the experimental results of acetonitrile or acetonitrile / water mixed solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com