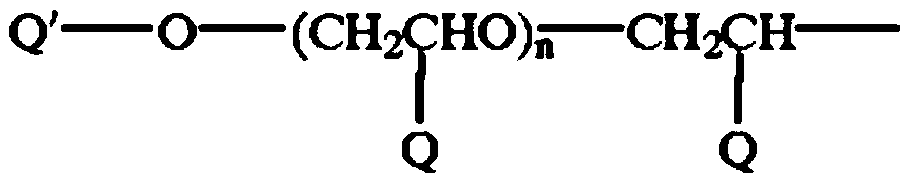A kind of yellow azo pigment composition and its preparation method and application
A technology of pigment composition and azo pigments, applied in the direction of pigment paste, organic dyes, etc., can solve the problems of pigment color influence, etc., and achieve the effects of batch stability guarantee, high degree of unitization, and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
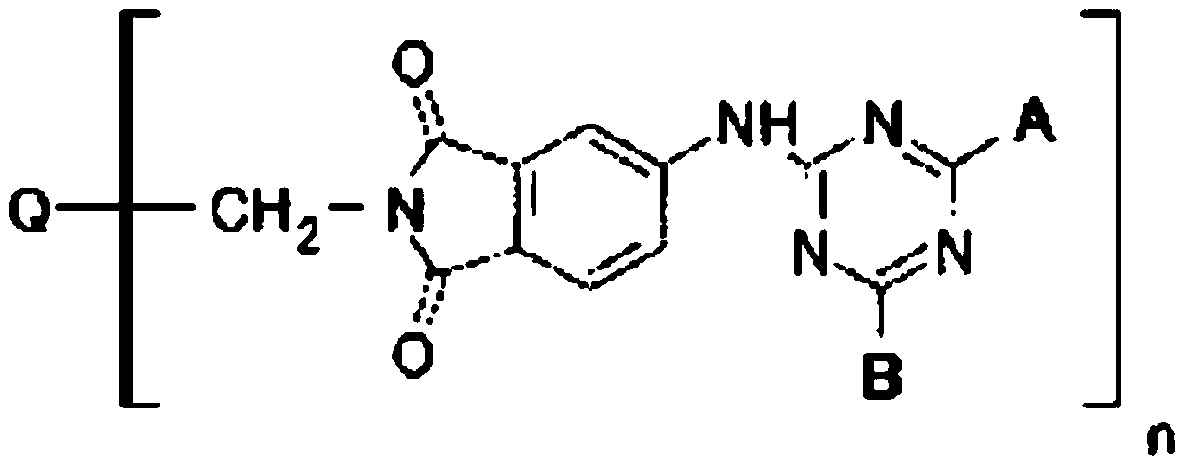
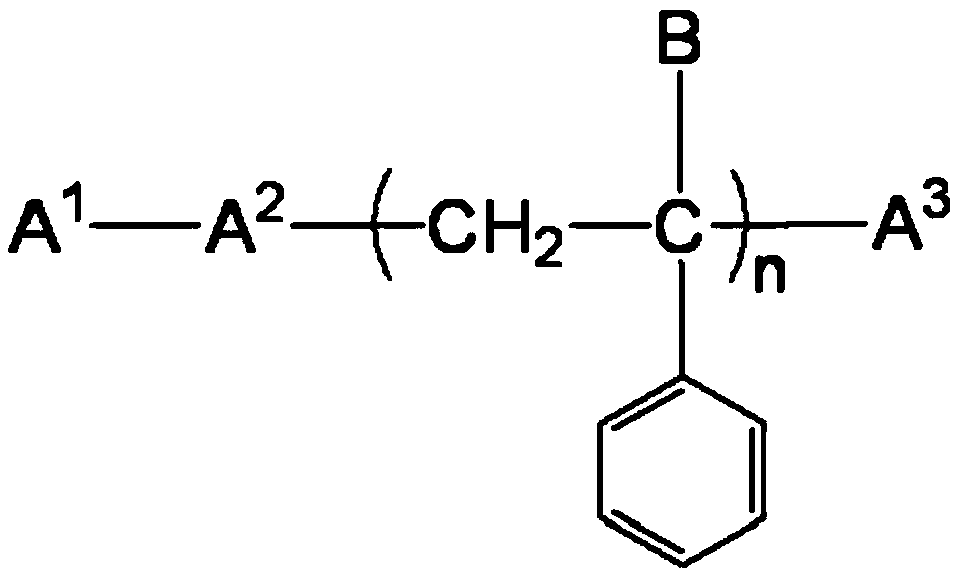
[0053] (-M of the engineered coupling component in the b component 1 -(M 2 )-R 1 p 1 R 2 q 1 )preparation:
[0054] 1) In a 1000ml there-necked flask with stirring, add 400g of ice water, add 36.90g (0.2mol) of cyanuric chloride, then slowly add 17.32g (0.1mol) of 4-aminobenzenesulfonic acid, dropwise add 20% of Aqueous sodium hydroxide solution to keep the pH value of the system at 3-5; when the pH value no longer changes, add polyetheramine (p2=35-45, q2=6-10, and molecular weight 2000) 100g (0.1mol), Heat to 60°C, add dropwise 20% aqueous sodium hydroxide solution to keep the pH value of the system at 3-5, and react for 1 hour; add 10.31 g (0.1mol) of diethylenetriamine, heat at 60°C for 1 hour and add dropwise 20% The pH value of the system is kept at 5-7; keep stirring for standby;
[0055] 2) In a 500 ml three-necked flask with stirring, add 200 g of chlorosulfonic acid and 20.72 g (0.1 mol) of o-methoxyacetoacetanilide, heat at 60° C. for 2 h, during which 20 g o...
Embodiment 2
[0058] Composition preparation:
[0059] 1) Preparation of diazonium salt: o-methoxy-p-nitroaniline (red base B) was slurried in 1.5 equivalent of 15% hydrochloric acid for 8 hours, then 1.5 equivalent of concentrated hydrochloric acid was added, and 1.02 equivalent of 1.02 equivalent was added dropwise at 0-5°C 30% aqueous sodium nitrite solution, followed by reaction with starch potassium iodide test paper to obtain diazonium salt solution;
[0060] 2) Coupling solution preparation: 99 parts of mol parts of o-methoxyacetoacetanilide and 1 part of mol parts of above-mentioned embodiment 1 are prepared to obtain and transform the coupling component to be dissolved in 1.2 equivalent sodium hydroxide solution, be mixed with 10 % concentration solution for use.
[0061] 3) In the acetic acid / sodium acetate buffer solution, synchronously add equimolar amounts of the solutions of step 1) and step 2) dropwise to ensure that the pH of the system is 4.4-4.6, and detect with H acid so...
Embodiment 3
[0069] Example 3 Synthesis of Pigment Yellow 73 Modified Pigment Composition
[0070] The operation method is the same as in Example 1, except that the o-methoxy-p-nitroaniline (red base B) of step 1 is changed to o-nitro-p-chloroaniline, and then the composition is prepared according to the process recorded in the examples. A pigment composition containing the a-component and the b-component is obtained, wherein the a-component and the b-component have the following structures.
[0071]
[0072]
[0073] Comparative Example 2 Synthesis of Pigment Yellow 73
[0074] The composition was prepared according to the preparation process of the composition described in Example 2, except that the component b was not added to the coupling solution in step 2, and the mol fraction of o-methoxyacetoacetanilide was 100 parts.
[0075]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com