A kind of chimeric antigen receptor and its application
A chimeric antigen receptor and antigen recognition technology, applied in the direction of antibody medical components, receptors/cell surface antigens/cell surface determinants, hybrid peptides, etc., to prolong the period of complete remission, reduce the probability of occurrence, and enhance immune monitoring effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation and identification of hCD19 protein
[0070] Prepare Hcd19 protein according to the following steps, as follows:
[0071] hCD19-fc: Human CD19 recombinant protein (Accession #AAH06338) was expressed in HEK293 cells. The sequence of Pro20-Lys291 in the coding region of human CD19 gene (the C-terminus was tagged with Fc) was selected for transfection. The purified protein was identified by SDS-PAGE gel.
[0072] hCD19-his: Human CD19 recombinant protein (Accession #P15391-1) was expressed in HEK293 cells. The human CD19 gene coding region Pro20-Lys291 sequence (His-tagged at the C-terminus) was selected for transfection. The purified protein was identified by SDS-PAGE gel.
Embodiment 2
[0073] Example 2 Antibody Preparation
[0074] (I) Antigen Conjugation and Immunization
[0075] (1) The hCD19-Fc recombinant protein was immunoconjugated with various MabSpace immune-enhancing peptides, and the conjugated protein was detected by SDS-PAGE gel;
[0076] (2) Freund's complete adjuvant (Pierce, Cat#77140) was added to the above conjugated hCD19-Fc protein at a ratio of 1:1 for emulsification, and then injected into H2L2 mice by subcutaneous and intraperitoneal injection respectively for immunization. H2L2 mice are produced by HarbourBioMed and carry genes for human variable regions and rat constant regions, and do not contain endogenous mouse antibody genes. Additional immunizations were performed using CpG (cytosine-phosphosulfoyl-guanine) and alum to preserve the native structure of the protein. After the first immunization and after the immunization (at least once every 2 weeks), the mouse serum was obtained, and the anti-hCD19 titer in the antiserum was a...
Embodiment 3
[0081] Example 3 Subcloning acquisition of positive hybridoma cells and small-scale antibody production
[0082] (1) positive hybridoma cell subclone
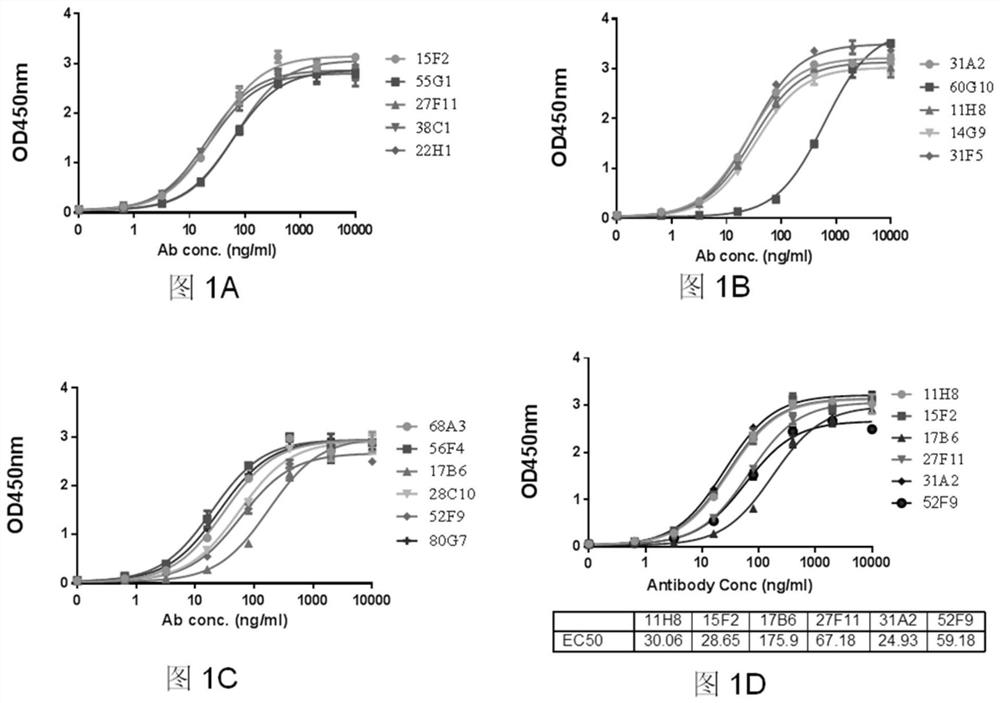
[0083] (1) Carry out gradient dilution of ELISA-positive hybridoma cells on a 96-well plate, and select cells with ideal affinity and blocking activity; after culturing for 7 days, form a cell clone group, collect the supernatant, and use the examples according to the antigen binding ability. 2 to further filter;
[0084] (2) Select the clone with the highest antigen affinity according to the screening result, and expand and cultivate in the hybridoma growth medium. After 7 days, the antigen-binding ability of the cultured supernatant of the hybridoma cells after screening was tested again; the subclone screening test was performed at least twice, until at least 90 wells (96-well plate);
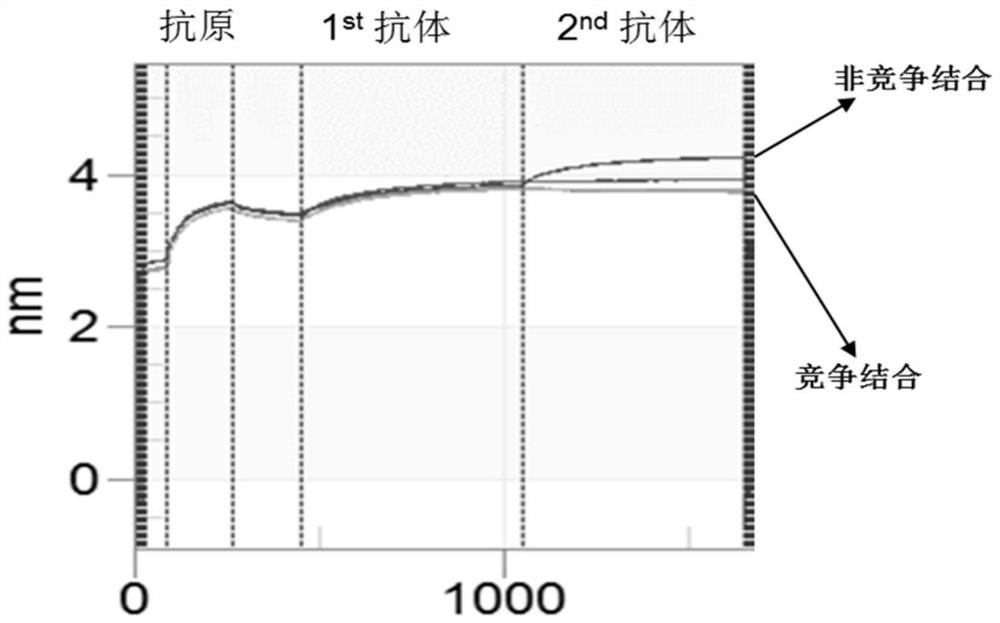
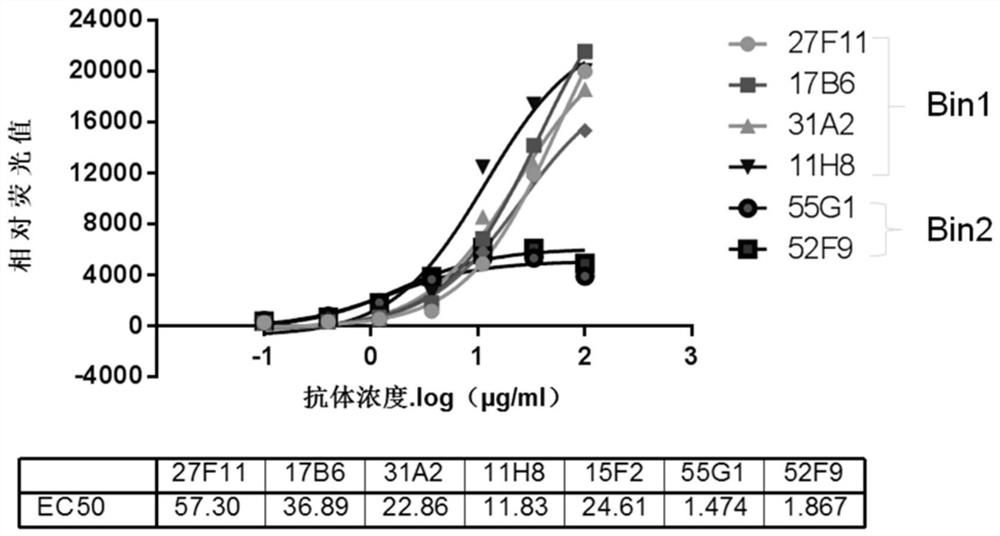
[0085] (3) When more than 90 wells showed a positive binding signal, identify the two clones with the highest antigen-binding activity a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com