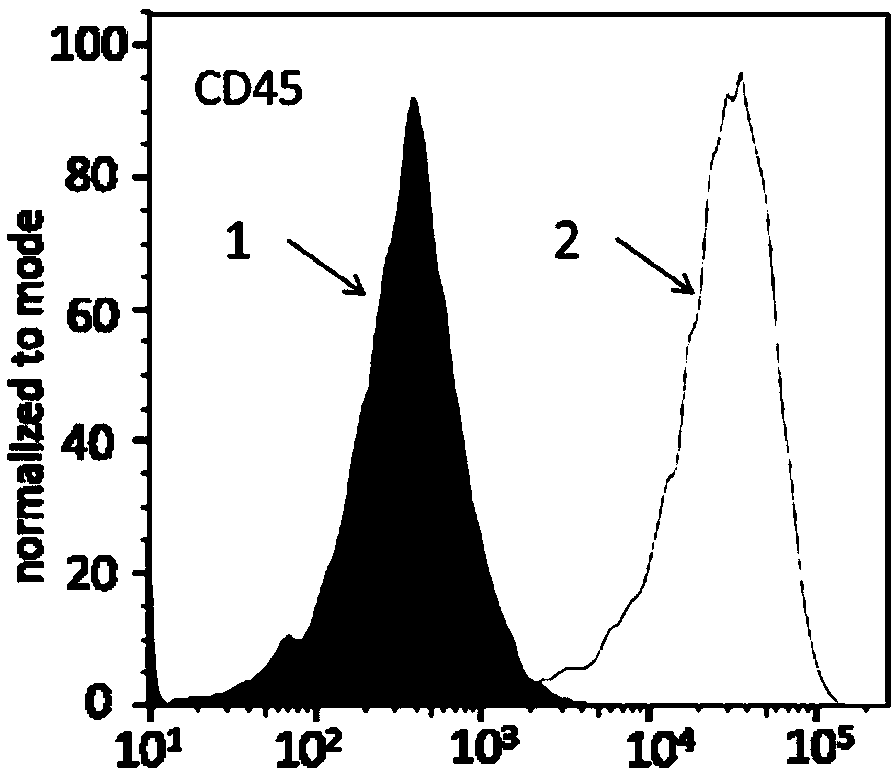
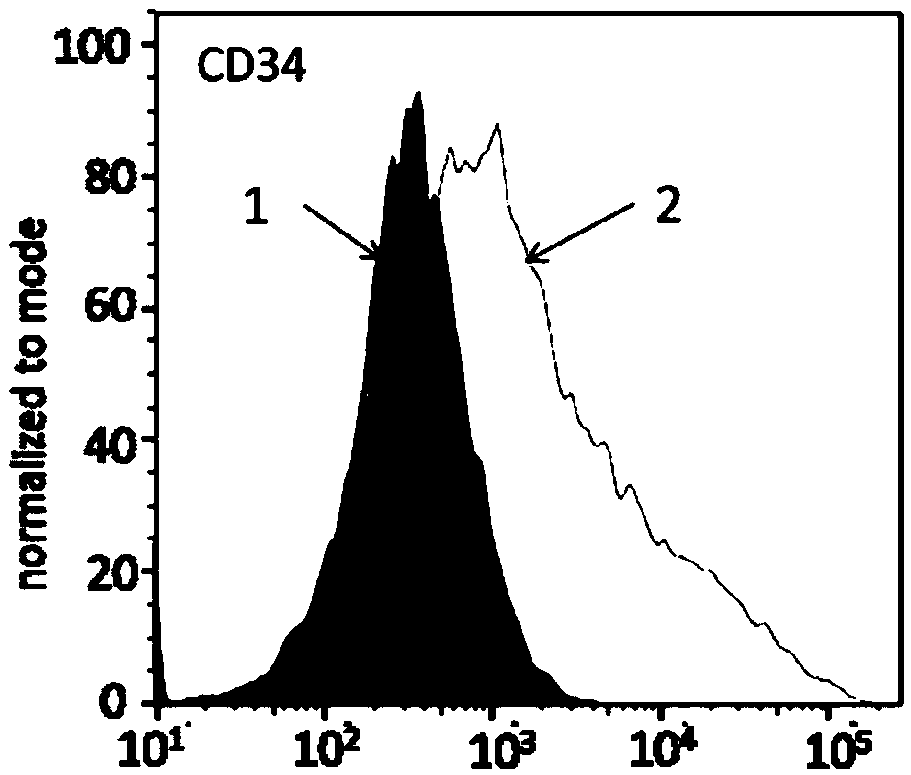
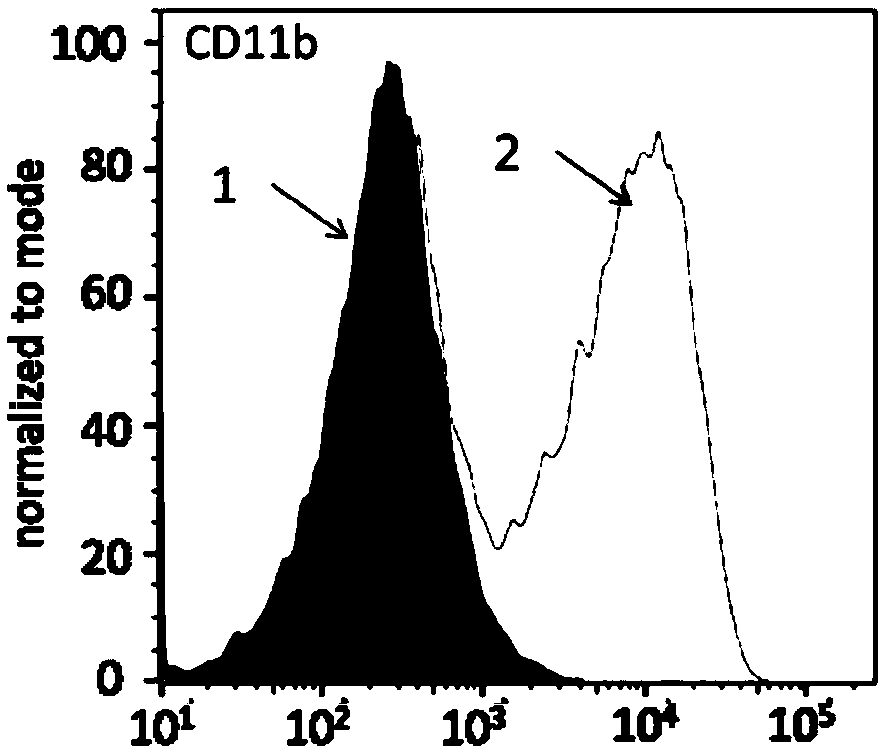
Macrophages capable of targeting tumor cells and preparation method thereof
A macrophage and tumor cell technology, applied in the field of macrophage and its preparation, can solve the problems of poor recognition ability and weak killing effect, and achieve the effect of easy entry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] A method for preparing macrophages capable of targeting tumor cells, expressing genes encoding chimeric antigen receptors in macrophages to obtain macrophages capable of targeting tumor cells. This method provides a new idea for immuno-oncology therapy.
[0103] In a preferred embodiment of the present invention, the preparation method further includes the step of preparing HLA-I gene-deficient macrophages.
[0104] In a preferred embodiment of the present invention, the preparation method further includes the step of preparing B2M gene-deficient macrophages.
[0105] In a preferred embodiment of the present invention, the preparation method includes directional differentiation of pluripotent stem cells to obtain macrophages capable of targeting tumor cells, and the pluripotent stem cells contain genes encoding chimeric antigen receptors.
[0106] In some embodiments, the pluripotent stem cells are HLA-I deficient pluripotent stem cells.
[0107] In some embodiments, ...
Embodiment 1
[0163] Example 1 Preparation of induced pluripotent stem cells
[0164]On day -1, 10ml of peripheral blood was drawn from the patient or volunteer, and PBMC (peripheral blood mononuclear cells) were separated with lymphocyte separation medium, cultured in H3000+CC100, and MEF cells (fibroblasts) were revived.
[0165] On day 0, take 1-2 million PBMCs and electroporate plasmids containing reprogramming factors OCT4, SOX2, KLF4, LIN28, and L-MYC. After electroporation, the cells are cultured in H3000+CC100 medium, centrifuged at 250 rcf for 5 min after 4 h, and discarded. The supernatant was resuspended with H3000+CC100 medium, and cultured in MEF cell plates.
[0166] On day 2, recover MEF cells.
[0167] On day 3, take the cell supernatant into a 15ml centrifuge tube, add 200ul Tryple to digest the adherent cells for 5min, stop the digestion with 1ml H3000, pipette and transfer to the corresponding centrifuge tube, centrifuge at 250rcf for 5min, discard the supernatant, and u...
Embodiment 2
[0172] Example 2 Recovery, culture and passage of 293T cells
[0173] (1) Resuscitation: Take out the frozen cells from the liquid nitrogen tank and quickly place them in a 37°C water bath, shake them quickly to melt them. Prepare a 15ml centrifuge tube in an ultra-clean bench, add 5ml of complete medium and the cells in the frozen tube, mix well, and centrifuge at 250rcf / min for 5min. Discard the supernatant, resuspend with 5ml of complete medium and transfer to T25 culture flask, 37°C, 5% CO 2 cultured in an incubator. Observe the cell survival rate the next day, discard the old medium, and add 5ml of fresh medium.
[0174] (2) Subculture: when the cells grow to 80%-90%, subculture. Discard the supernatant, add 5ml of PBS and shake gently, discard the PBS, add 1ml of 0.25% trypsin, digest for 10s to 20s until the cells become round and the intercellular space becomes larger, add 3ml of complete medium, mix well, transfer to a 15ml centrifuge tube, and centrifuge 250rcf / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com