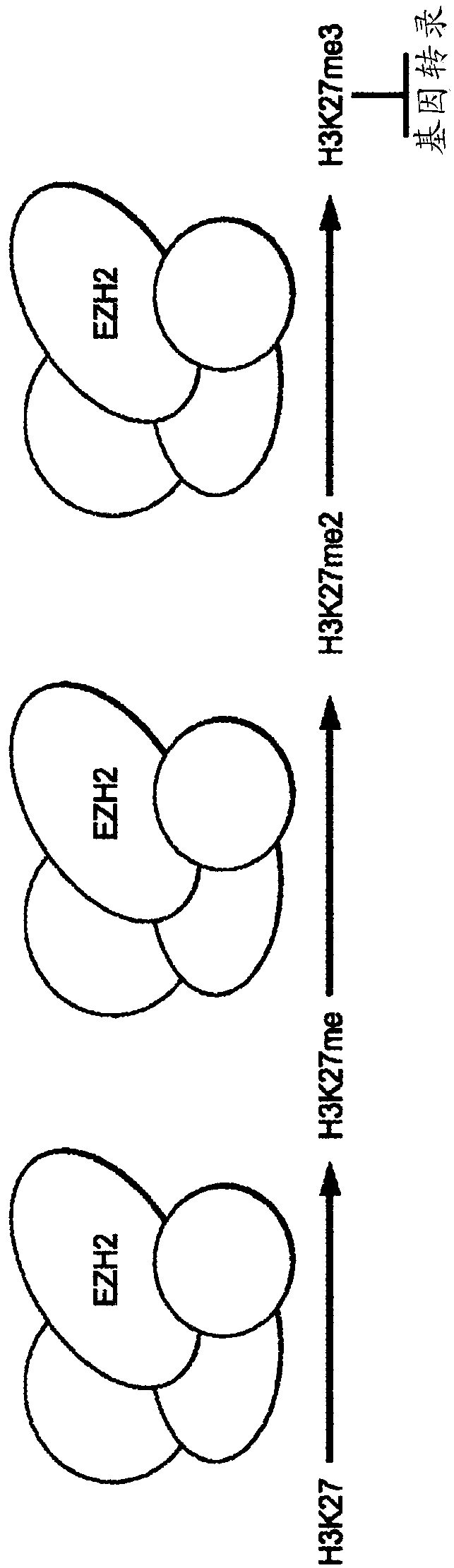
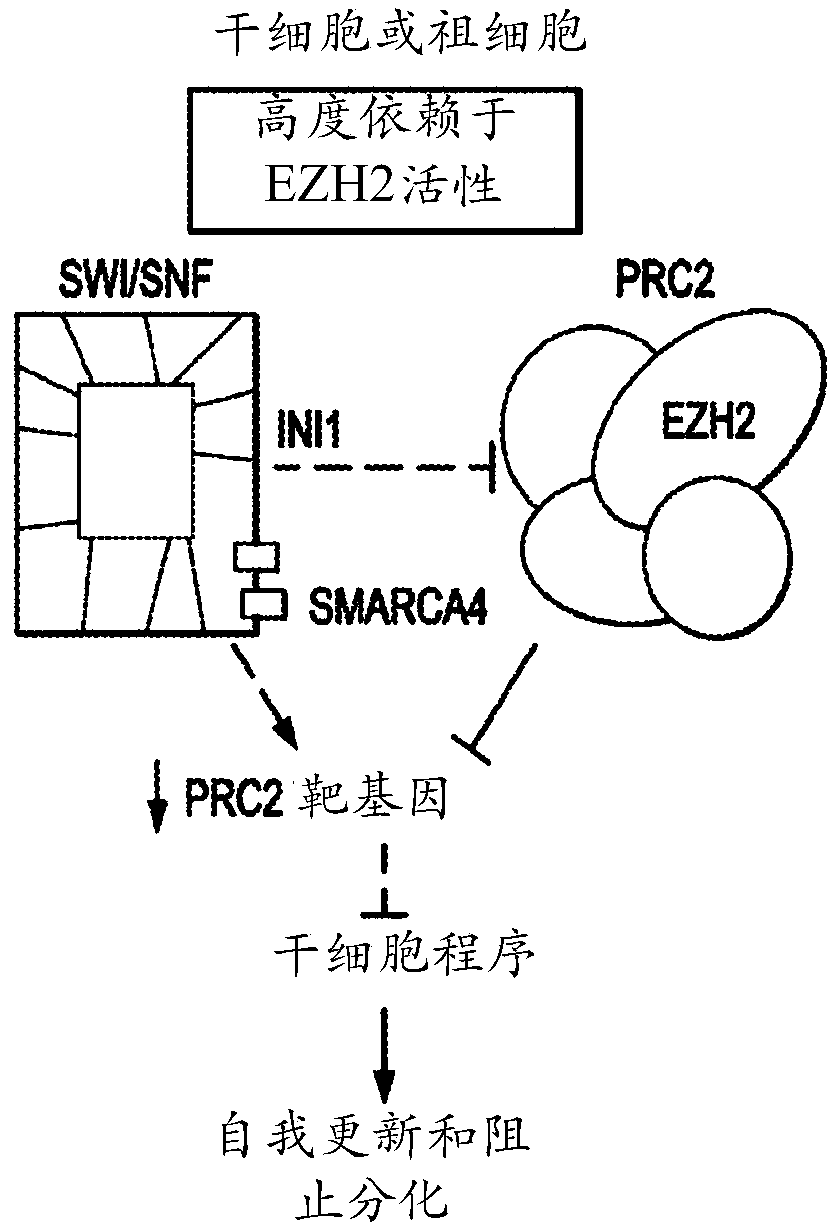
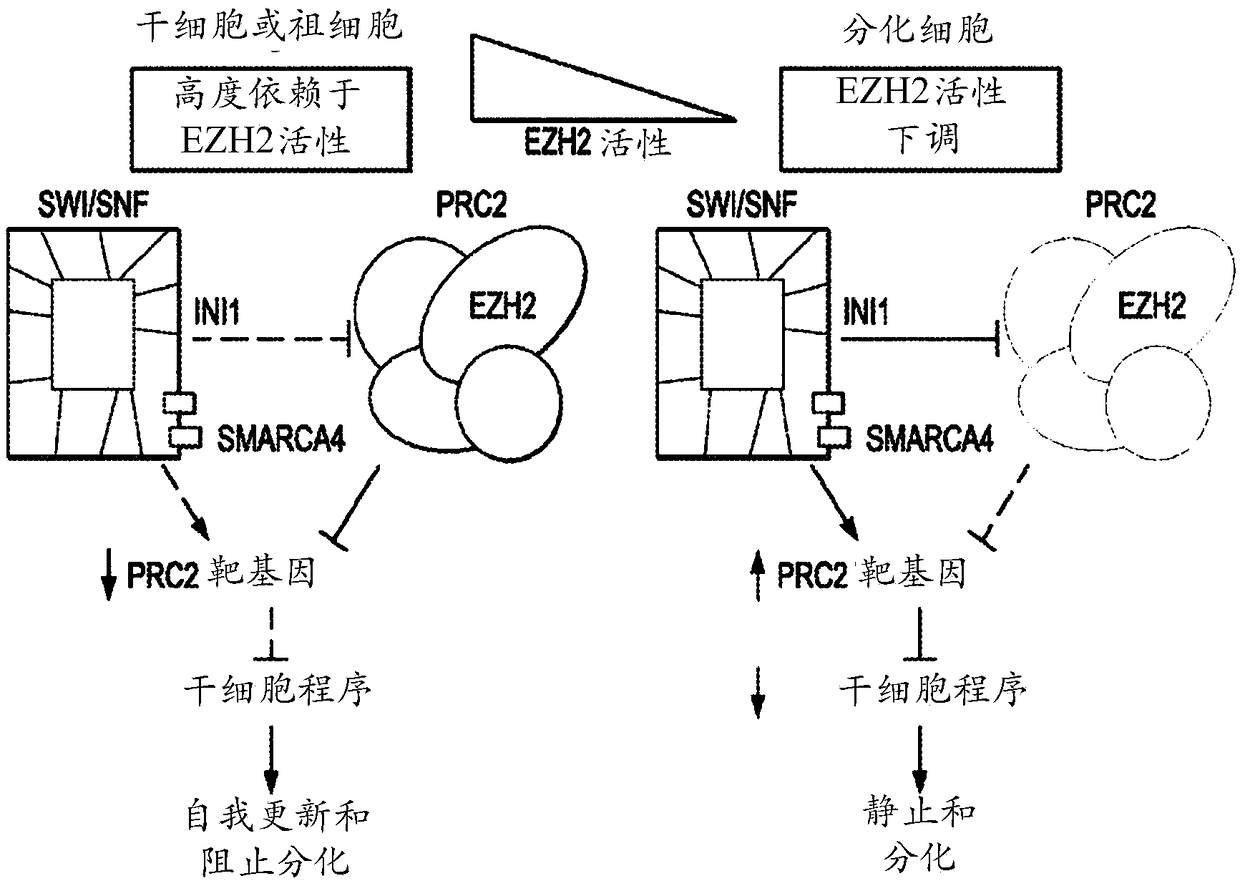
Method of treating malignant rhabdoid tumor of the ovary (MRTO)/small cell cancer of the ovary of the hypercalcemic type (SCCOHT) with an EZH2 inhibitor
A malignant rhabdoid, hypercalcemia technique for the treatment of malignant rhabdoid tumor of the ovary (MRTO)/small cell carcinoma of the ovary hypercalcemia (SCCOHT) with EZH2 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0158] In order that the invention disclosed herein may be more effectively understood, the following examples are provided. It should be understood that these examples are for illustrative purposes only and should not be construed as limiting the disclosure in any way.
example 1
[0159] Example 1: Treatment of SMARCA4-negative MRTO / SCCOHT with tazemetostat
[0160] A 27-year-old human female diagnosed with SMARCA4-negative MRTO / SCCOHT was successfully treated with oral tablet twice-daily (BID) administration of 1600 mg EPIZ-6438 (Tazemetostat). Tumor size decreased from baseline after 8 weeks of treatment and further decreased from 8-week measurements after 16 weeks of treatment.
[0161] The subject was diagnosed with SMARCA4-negative MRTO / SCCOHT in 2013. Throughout 2014, subjects were treated with a course of cisplatin / cyclophosphamide / doxorubicin / etoposide followed by a course of carboplatin / etoposide / cyclophosphamide. None of the treatments were successful. The subject then underwent autologous hematopoietic cell transplantation, which also failed to treat SMARCA4-negative MRTO / SCCOHT.
[0162] Subjects are currently undergoing therapy with 1600 mg tazemetostat administered twice daily (BID) with oral tablets. Figure 6A Preliminary results are...
example 2
[0163] Example 2: Remission of INI1 and SMARCA4 negative tumors
[0164] Treatment of INI1 and SMARCA4-negative tumors with tazemetostat induces pharmacodynamic inhibition of HeK27me3 in tumor tissues.
[0165] Evaluation of MRT and MRTO / SCCOHT clinical activity after tazemetostat treatment showed stable disease for at least 6 months, partial response or complete response.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com