A kind of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate esterification reaction catalyst and its preparation method and application
A technology of pentanediol diisobutyrate and esterification reaction, which is applied in the preparation of carboxylate, catalysts for physical/chemical processes, chemical instruments and methods, etc. It can overcome the problems of serious equipment corrosion, high product quality and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
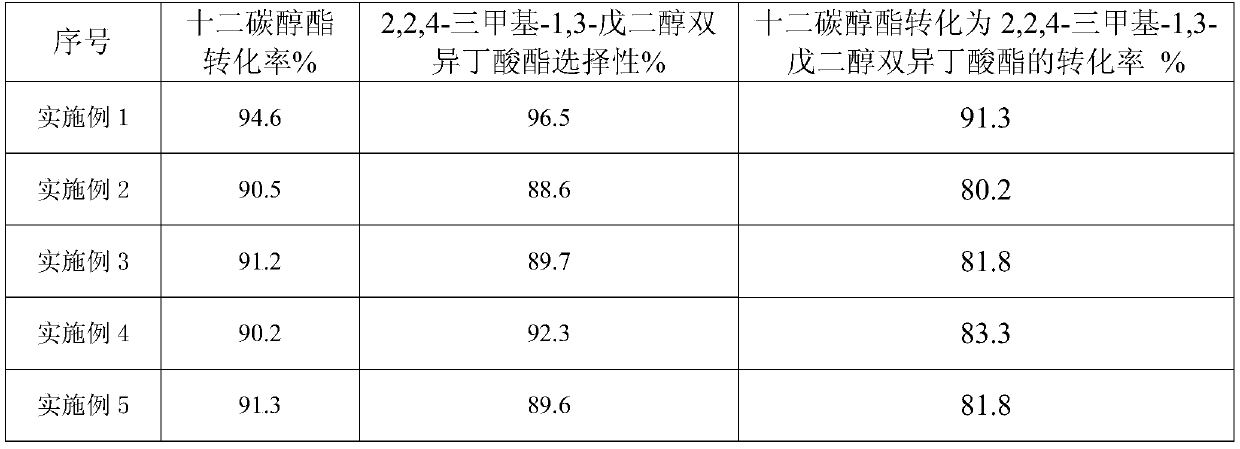
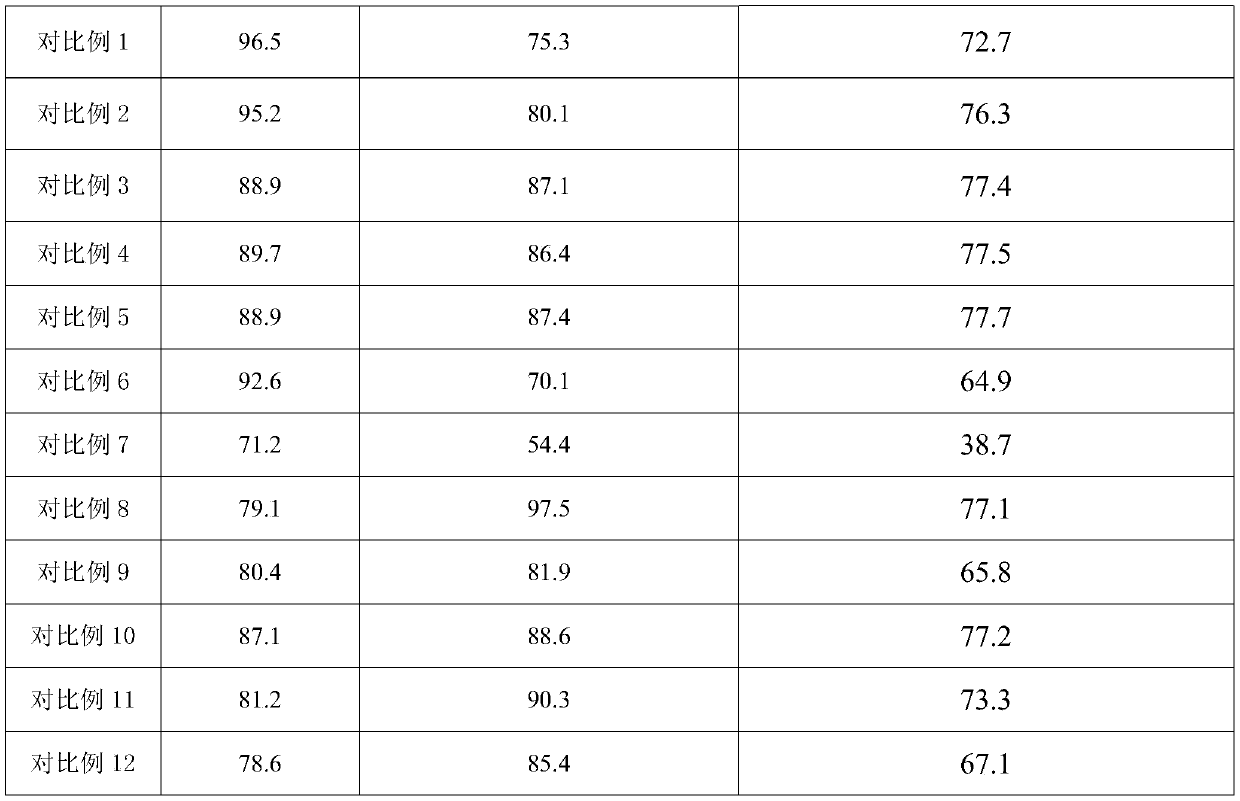
Examples
Embodiment 1
[0018] Take 10 grams of NaHSO 4 ·H 2 O and 50 g NaH 2 PO 4 , adding deionized water while stirring to prepare a saturated mixed salt solution, heating and evaporating the saturated solution at 150°C until the mixed salt is completely crystallized to obtain NaHSO 4 ·H 2 O-NaH 2 PO 4 Mixed inorganic salt catalyst.
[0019] 3 g NaHSO 4 ·H 2 O-NaH 2 PO 4 , 100 grams of isobutyric acid and 100 grams of dodecyl alcohol ester were added to a three-necked flask and stirred, the reaction temperature was raised to 155°C so that it did not fluctuate, condensed and refluxed for 8 hours and water was discharged, and the catalyst was filtered and separated after the reaction was completed. The isobutyric acid was separated by rotary evaporation under vacuum condition at ℃ to obtain 2,2,4-trimethyl-1,3-pentanediol diisobutyrate. The catalyst separated by filtration was repeated 6 times according to the above operation steps.
Embodiment 2
[0021] NaHSO4 H2O-KH2PO4 instead of NaHSO in Example 1 4 ·H 2 O-NaH 2 PO 4 , the filtered catalyst is not reused, and other steps are the same as in Example 1.
Embodiment 3
[0023] NaHSO4 H2O-NH4H2PO4 instead of NaHSO in Example 1 4 ·H 2 O-NaH 2 PO 4 , the filtered catalyst is not reused, and other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com