igm semi-quantitative leptospirosis diagnostic kit
A diagnostic kit and technology for spirochetosis, applied in measuring devices, biological tests, instruments, etc., can solve the problems of diagnosing patients with leptospirosis and not distinguishing leptospirosis epidemic areas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2-3
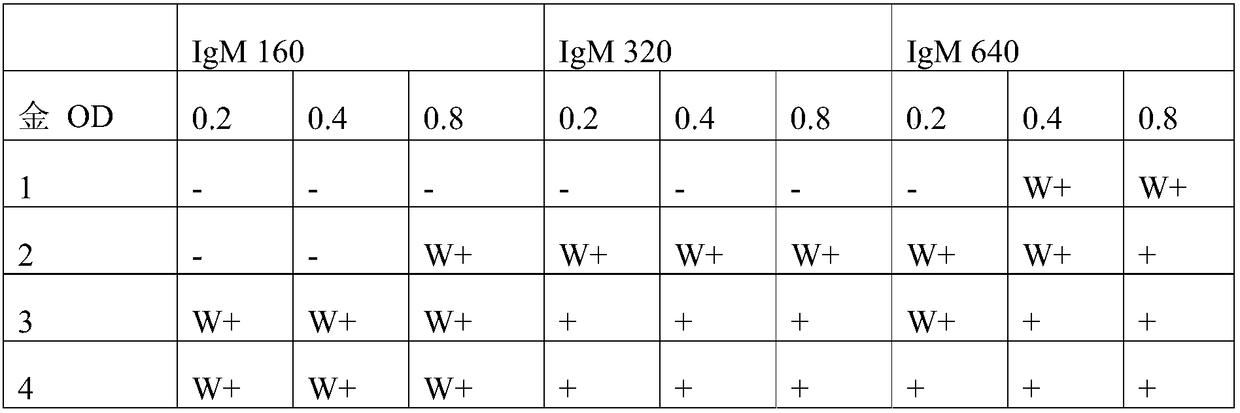
[0037] According to one embodiment, said first kit further comprises a gold-conjugated-goat-anti-human IgM antibody with an OD of 3.5 to 4.5, and said second kit may further comprise a gold-conjugated-anti-human IgM antibody with an OD of 1.5 to 2.5. Goat-anti-human IgM antibody. When the gold-conjugated-goat-anti-human IgM antibody of the first kit is contained at an OD of less than 3.5, the color development due to the antigen-antibody reaction may not be displayed well, and when the gold-conjugated-goat-anti-human IgM antibody is contained at an over OD 4.5, Samples that can have a MAT Ab titer below the cutoff value of 50 can lead to problems with positivity. In addition, when the gold-conjugated-goat-anti-human IgM antibody of the second kit is included at an OD of less than 1.5, the color development due to the antigen-antibody reaction may not be displayed well, and is exceeded at an OD of more than 2.5 Sometimes, there may be a problem with samples having a MAT Ab tit...
Embodiment 1
[0046] Example 1: Production of polysaccharide antigen derived from Leptospirosis
[0047] 1-1. Cultivation of Leptospirosis strains
[0048] After the EMJH medium base (Leptospira medium base Ellinghausen McCullough Hohnson Harris) was melted to 0.23g / 100ml and sterilized, 10ml of EMJH medium supplement was added to produce the medium. Leptospirosis bacteria were inoculated on this medium, and shaking culture was carried out at 24° C. for five days.
[0049] 1-2. Production of leptospirosis antigen
[0050] The Leptospirosis bacteria cultured in EMJH medium for five days were separated at 4080×g for 20 minutes, and the thalline was treated with 1XPBS slow agent (buffer) (137mM NaCl, 2.7mM KCl, 10mM NaCl 2 HPO 4 , 2mM KH 2 PO 4 ) and washed three times, heated at 100°C for one hour. Among them, make proteolytic enzyme (Proteinase) K become For treatment, the reaction was performed overnight at 37°C. Thereafter, EGTA was added at 2 mM, reacted at 70° C. for 15 minutes,...
Embodiment 2
[0051] Embodiment 2: making diagnostic kit
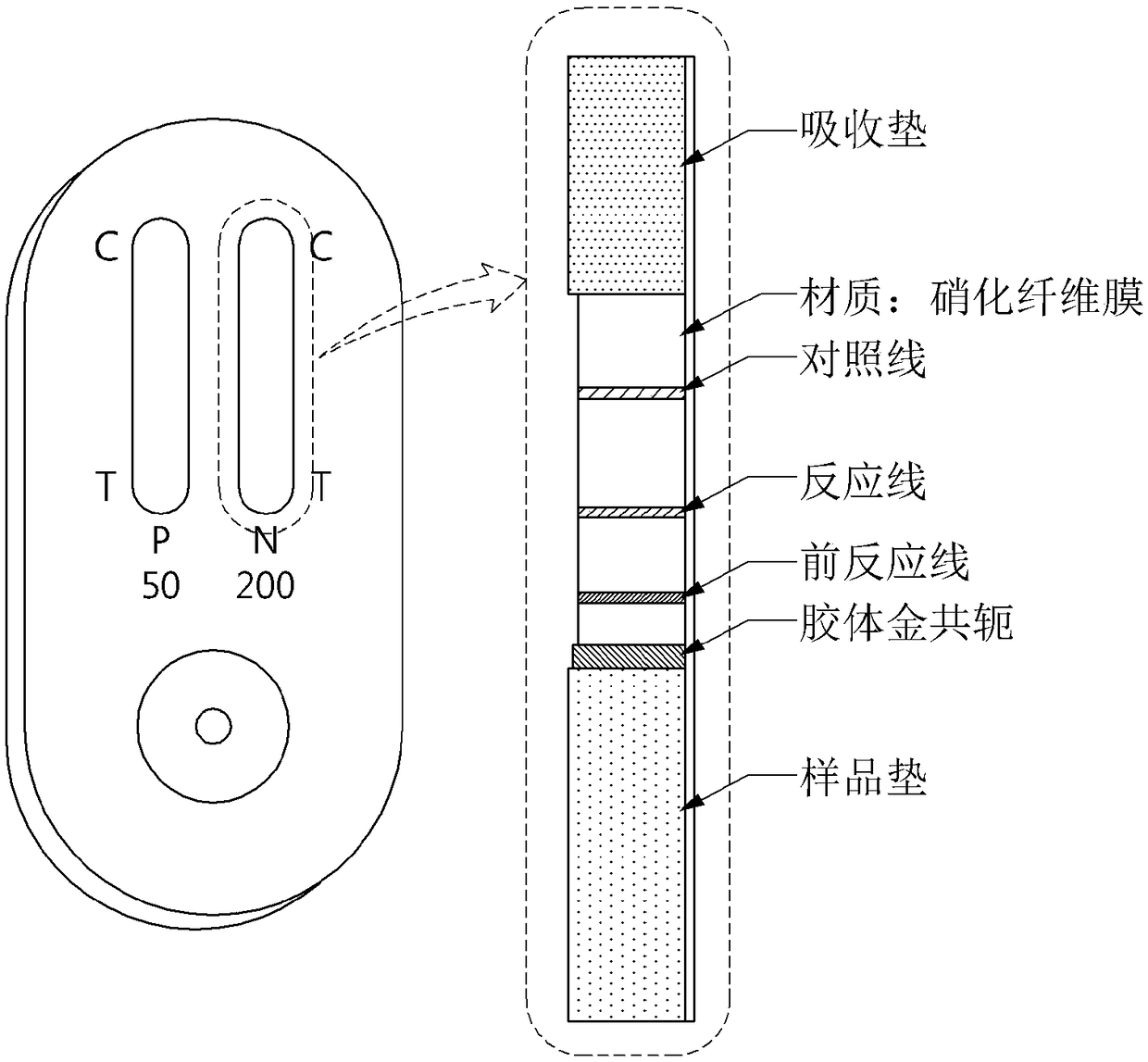
[0052]The NC membrane of the plastic backing (backing) is used as the basic structure, the antigen is fixed in the middle, the glass fiber membrane (conjugated mat) that fits in the storage is attached to the lower part, and the fibrous membrane that absorbs the excess aqueous solution is attached to both ends ( Sample pads, absorbent pads) are composed of interconnected forms. The glass fiber membrane at the lower end has strong absorption, not only can absorb the sample in a short time, but also the dissolved neutralization body moves before the upper immunostrip, prolonging the stagnation time, which can induce an effective reaction between the analyte and the neutralization body. The immunostrip in the middle is spatially separated and fixed, and the analyte can be sensed to measure the antigen (test line or capture line) and the specific antibody of the complex (control line), and the absorbent pad located at the upper end of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com