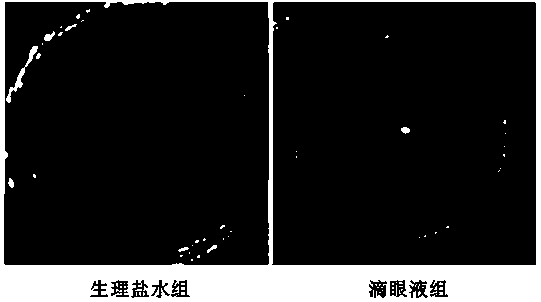
Application of Tripterygium in Preparation of Anti-Keratoplasty Rejection Eye Drops
A technology of triptolide and transplant rejection, which is applied in the intersection of biomedical materials and medicine, can solve the problems of undiscovered ophthalmic diseases, limited clinical application and curative effect, poor water solubility, etc., and achieves anti-corneal transplant rejection and high encapsulation. rate, the effect of high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
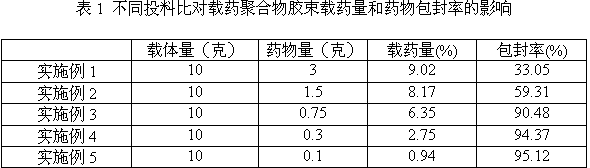
[0014] Weigh 10.0 g of ternary block copolymer PEG-PCL-PEI and 3.0 g of tripterine, and dissolve them together in 20 mL of a mixed solvent consisting of methanol and acetonitrile at a volume ratio of 1:1; then, under ultrasonic stirring, Add the mixture dropwise to 50mL of water for injection, vacuum distill to remove the organic solvent, and then pass through an ultrafiltration device (molecular weight cut-off: 100,000 Da) for 3 times to remove free tripterine to obtain drug-loaded polymer micelles , adding water for injection to make the volume to 100mL, to obtain tripterine eye drops.
Embodiment 2
[0016] The difference from Example 1 is: 10.0 g of PEG-PCL-PEI, 1.5 g of tripterine, and the others are the same as in Example 1.
Embodiment 3
[0018] The difference from Example 1 is: 10.0 g of PEG-PCL-PEI, 0.75 g of tripterine, and the others are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com