Preparation method of pulegone derivative
A technology of menthone and derivatives, which is applied in the field of derivatization and preparation of 5-methyl-2-(1-methylethylene)cyclohexanol, which can solve the problems of expensive and difficult raw material sources, and achieve product yield High, good optical purity, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
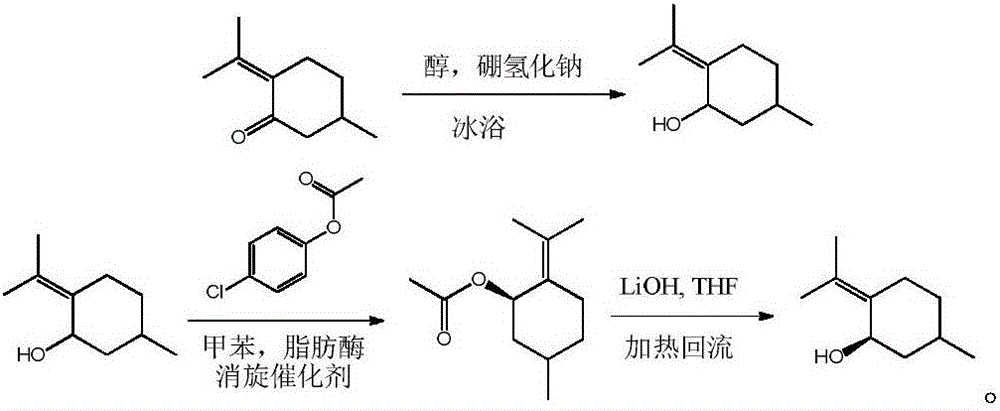
[0010] 1) At 0°C, add 250ml of anhydrous methanol and 15.2g of menthone to a single-necked flask, stir for 15 minutes, then add 11.3g of sodium borohydride. After feeding, seal the flask with a balloon and keep it at 0°C for 3 hours , point the board to detect that the long-leaved menthol has reacted completely, stop the reaction; dilute the sodium borohydride with hydrochloric acid solution until no bubbles emerge, distill methanol and extract three times with 100ml dichloromethane, combine dichloromethane, dry, After concentration, 14.3 g of 5-methyl-2-(1-methylethylene)cyclohexanol was obtained, with a yield of 92.6%.
[0011] 2) In a constant temperature shaker, with a 200ml blue bottle as a reaction vessel, add 60ml of toluene, 7.7g of 5-methyl-2-(1-methylethylene) cyclohexanol, 10.0g of p-chlorophenol B Ester, 0.3g of porcine pancreatic lipase PPL, 1.5g of acid resin D006, after feeding, the temperature was raised to 35°C for reaction, after 11 hours, the detection of 5-...
Embodiment 2
[0015] 1) At 0°C, add 1000ml of anhydrous methanol and 152g of menthone to a single-necked flask, stir for 20 minutes, then add 150g of sodium borohydride, seal the flask with a balloon, keep at 0°C for 4 hours, TLC Detect that the long-leaf menthol has reacted completely, stop the reaction; dilute the sodium borohydride with hydrochloric acid solution until no more bubbles emerge, distill the methanol and extract three times with 300ml ethyl acetate, combine the ethyl acetate, dry, and concentrate 143.8 g of 5-methyl-2-(1-methylethylene)cyclohexanol was obtained, with a yield of 93.4%.
[0016] 2) In a constant temperature shaker, with a 1000ml blue cap bottle as a reaction vessel, add 700ml of toluene, 77g of 5-methyl-2-(1-methylethylene) cyclohexanol, and 110g of p-chlorophenol acetate , 3g of porcine pancreatic lipase PPL, 10g of acidic resin D006, after feeding, the temperature was raised to 40°C for reaction. After 12 hours, it was detected that 5-methyl-2-(1-methylethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com