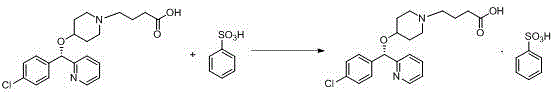
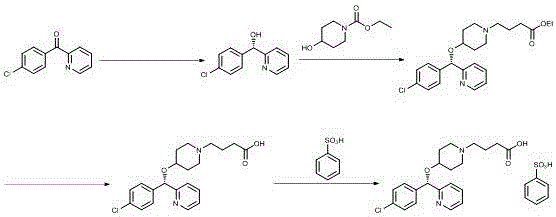
Salt-forming method of bepotastine besilate
A technology of shellfish benzenesulfonate and water benzenesulfonic acid, which is applied in the field of pharmaceutical raw materials and its synthesis, can solve the problems of unsuitability for industrial production, long crystallization time, and difficulty in removal, and achieve high product yield and long crystallization time The effect of shortening and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one: the preparation of bepotastine besilate
[0029] Add benzenesulfonic acid monohydrate (35.51g) into isopropanol (35ml), stir until completely dissolved, and set aside. Then (+)-(S)-4-{4-[(4-chlorophenyl)(2-pyridyl)methoxy]piperidinyl}n-butyric acid (80.00g) was dissolved in 240ml of isopropanol In the process, the temperature is controlled at 0° C. and the stirring speed is 120 rpm, a small amount of bepotastine benzenesulfonate seed crystals are added, and the above-mentioned isopropanol benzenesulfonate solution is added dropwise. After dropping, continue to insulate and stir for 3 hours before filtering, and the filter cake is rinsed with isopropanol. The wet product was dried under reduced pressure at 50-60°C to obtain 102.30 g of bepotastine besilate, yield: 93.49%. Submitted for inspection, the purity is 99.51%, the maximum impurity is 0.07%, and the residue on ignition is not more than 0.2%.
Embodiment 2
[0030] Embodiment two: the preparation of bepotastine besilate
[0031] Add benzenesulfonic acid monohydrate (35.48g) into ethanol (35ml), stir until completely dissolved, set aside. Then (+)-(S)-4-{4-[(4-chlorophenyl)(2-pyridyl)methoxyl]piperidinyl}n-butyric acid (80.03g) was dissolved in 240ml of ethanol, Control the temperature at 0° C. and the stirring speed at 120 rpm, add a small amount of bepotastine benzenesulfonate seed crystals, and add the above ethanol benzenesulfonate solution dropwise. After dropping, continue to insulate and stir for 3 hours before filtering, and the filter cake is rinsed with ethanol. The wet product was dried under reduced pressure at 50-60°C to obtain 100.67g of bepotastine besilate, yield: 92.01%. Submitted for inspection, the purity is 99.21%, the maximum impurity is 0.10%, and the residue on ignition is not more than 0.2%.
Embodiment 3
[0032] Embodiment three: the preparation of bepotastine besilate
[0033] Add benzenesulfonic acid monohydrate (35.55g) into methanol (35ml), stir until completely dissolved, and set aside. Then (+)-(S)-4-{4-[(4-chlorophenyl)(2-pyridyl)methoxyl]piperidinyl}n-butyric acid (80.01g) was dissolved in methanol 240ml, Control the temperature at 0° C. and the stirring speed at 120 rpm, add a small amount of bepotastine benzenesulfonate seed crystals, and add the above-mentioned methanol solution of benzenesulfonate dropwise. After dropping, continue to insulate and stir for 3 hours before filtering, and the filter cake is rinsed with ethanol. The wet product was dried under reduced pressure at 50-60°C to obtain 98.70 g of bepotastine besilate, yield: 90.21%. Submitted for inspection, the purity is 99.28%, the maximum impurity is 0.09%, and the residue on ignition is not more than 0.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com