Method for preparing linagliptin intermediate for treating II-type diabetis
A technology for intermediates and diabetes, which is applied in the field of drug synthesis, can solve the problems of increased risk, difficult handling, and increased post-processing workload, etc., and achieves the effects of mild conditions, good selection, and avoidance of dangerous and toxic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
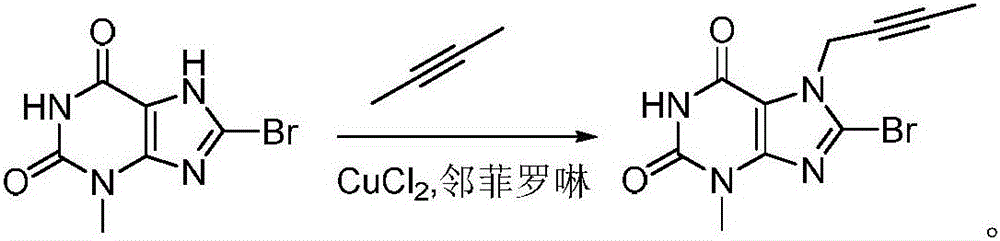
[0022] A method for preparing a linagliptin intermediate for the treatment of type II diabetes, comprising the following steps:
[0023] Under nitrogen protection, 8.1g (60mmol) of copper chloride, 7.2g (40mmol) of o-phenanthroline and 8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione 24.5g (100mmol) was dissolved in 150ml DMF, then the DMF solution of 2-butyne (containing 2-butyne 8.6g, 160mmol) was added dropwise, reacted at 40°C for 5 hours, then the reaction solution was poured into water, extracted with DCM, Wash with saturated brine, dry over anhydrous sodium sulfate, concentrate, then recrystallize in a mixed solvent of petroleum ether and DCM with a volume ratio of 40:1, filter, and dry to obtain the intermediate 8-bromo-3,7- Dihydro-3-methyl-9-(2-butynyl)-1H-purine-2,6-dione 26.8g, yield 90.2%, purity 99.92% (HPLC area normalization method).
Embodiment 2
[0025] A method for preparing a linagliptin intermediate for the treatment of type II diabetes, comprising the following steps:
[0026] Under nitrogen protection, 9.4g (70mmol) of copper chloride, 7.2g (40mmol) of o-phenanthroline and 8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione 24.5g (100mmol) was dissolved in 150ml DMF, then the DMF solution of 2-butyne (containing 2-butyne 7.6g, 140mmol) was added dropwise, reacted at 50°C for 6 hours, then the reaction solution was poured into water, extracted with DCM, Wash with saturated brine, dry over anhydrous sodium sulfate, concentrate, then recrystallize in a mixed solvent of petroleum ether and DCM with a volume ratio of 45:1, filter, and dry to obtain the intermediate 8-bromo-3,7- Dihydro-3-methyl-9-(2-butynyl)-1H-purine-2,6-dione 27.1 g, yield 91.1%, purity 99.97% (HPLC area normalization method).
Embodiment 3
[0028] A method for preparing a linagliptin intermediate for the treatment of type II diabetes, comprising the following steps:
[0029] Under nitrogen protection, 6.7g (50mmol) of copper chloride, 9g (50mmol) of o-phenanthroline and 24.5 g of 8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione g (100mmol) was dissolved in 150ml DMF, then the DMF solution of 2-butyne (containing 2-butyne 9.7g, 180mmol) was added dropwise, reacted at 45°C for 5 hours, then the reaction solution was poured into water, extracted with DCM, saturated Wash with brine, dry over anhydrous sodium sulfate, concentrate, then recrystallize in a mixed solvent of petroleum ether and DCM with a volume ratio of 40:1, filter, and dry to obtain the intermediate 8-bromo-3,7-di Hydrogen-3-methyl-9-(2-butynyl)-1H-purine-2,6-dione 26.7g, yield 89.7%, purity 99.89% (HPLC area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com