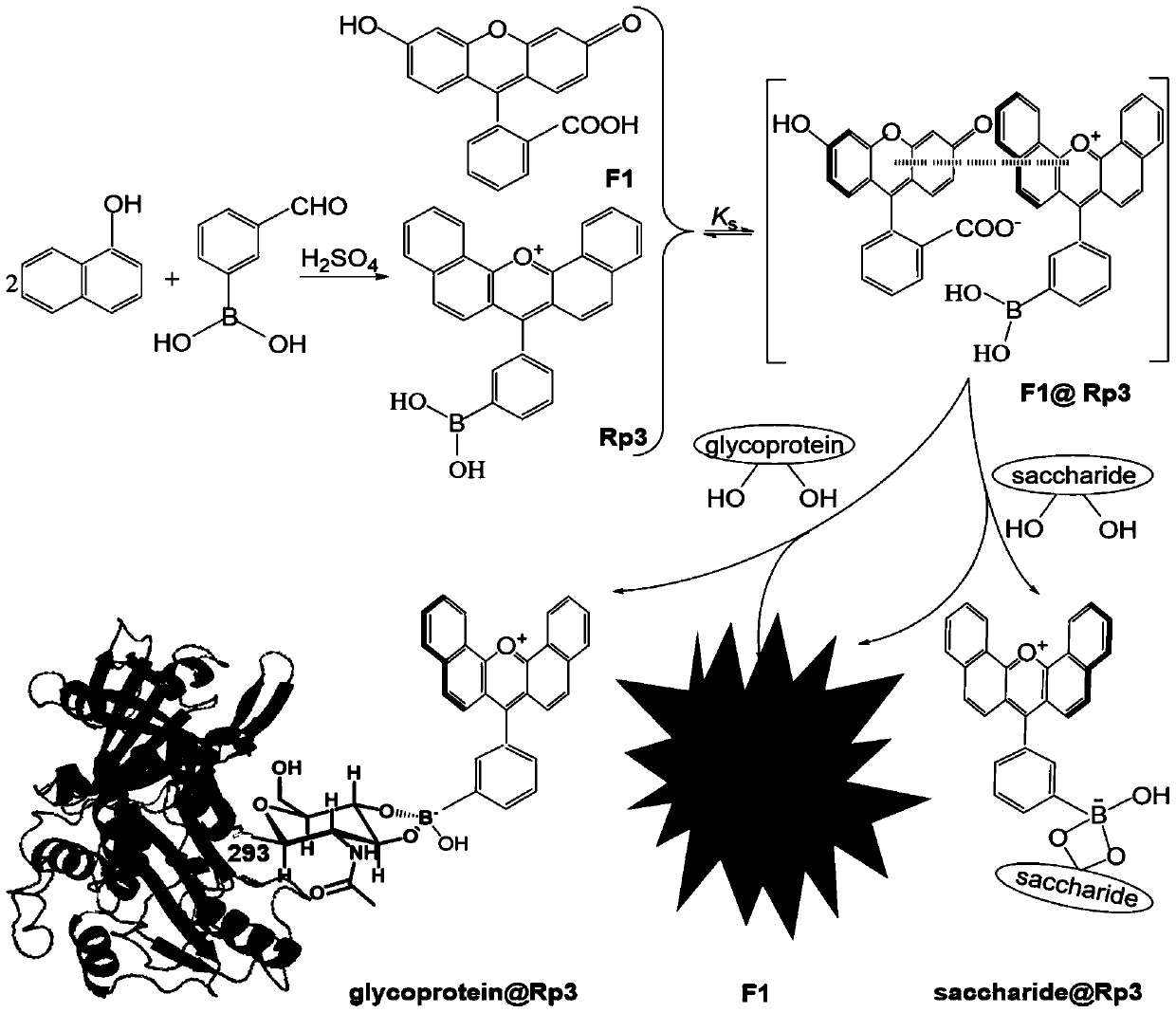
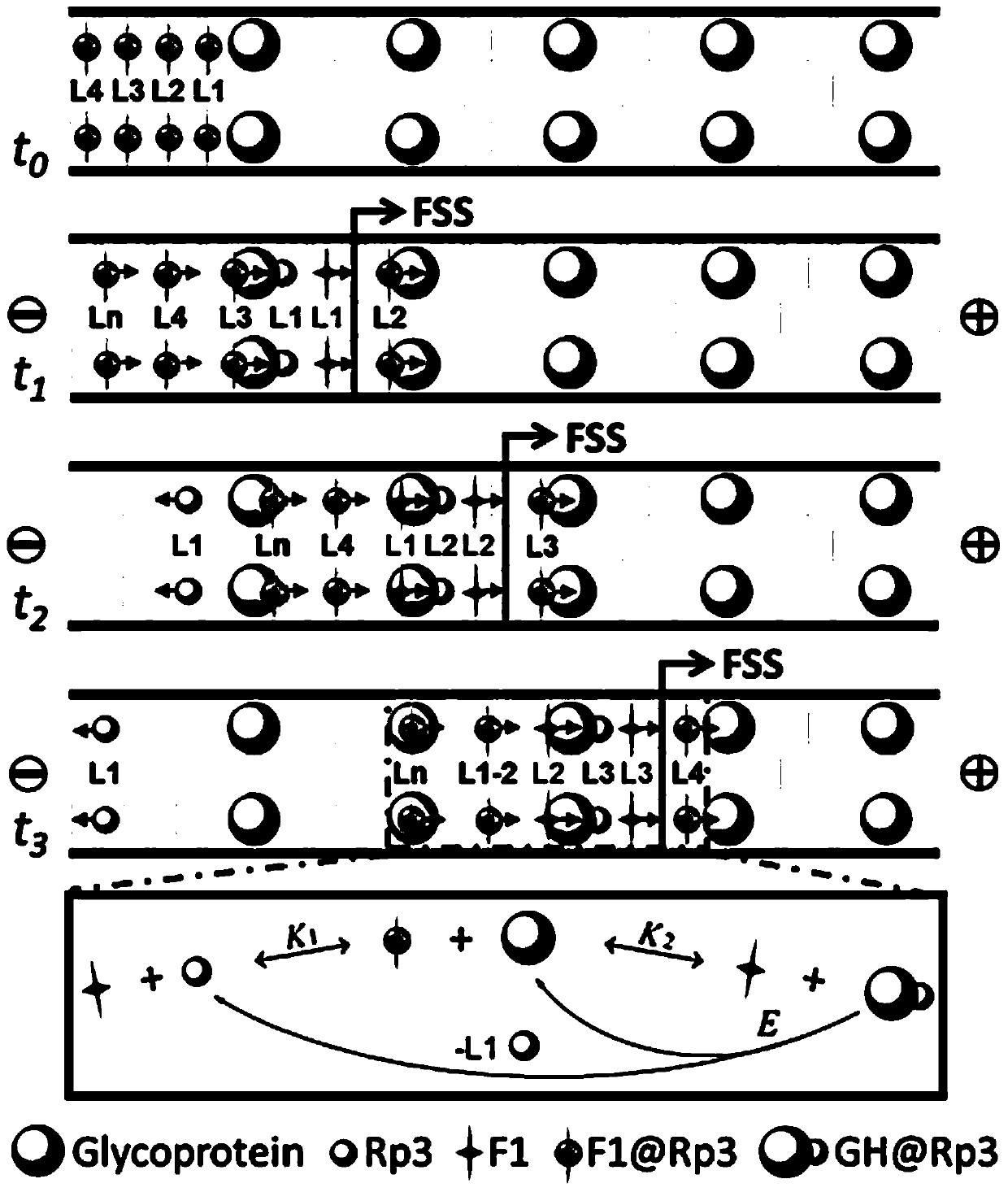
Fluorescent Velocity Sensing Method for Quantitative Detection of Glycosylated Proteins
A glycosylated protein and speed sensing technology, applied in the field of biomedicine, can solve the problems of large error value, poor detection stability and poor versatility, and achieve the effect of fast detection speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
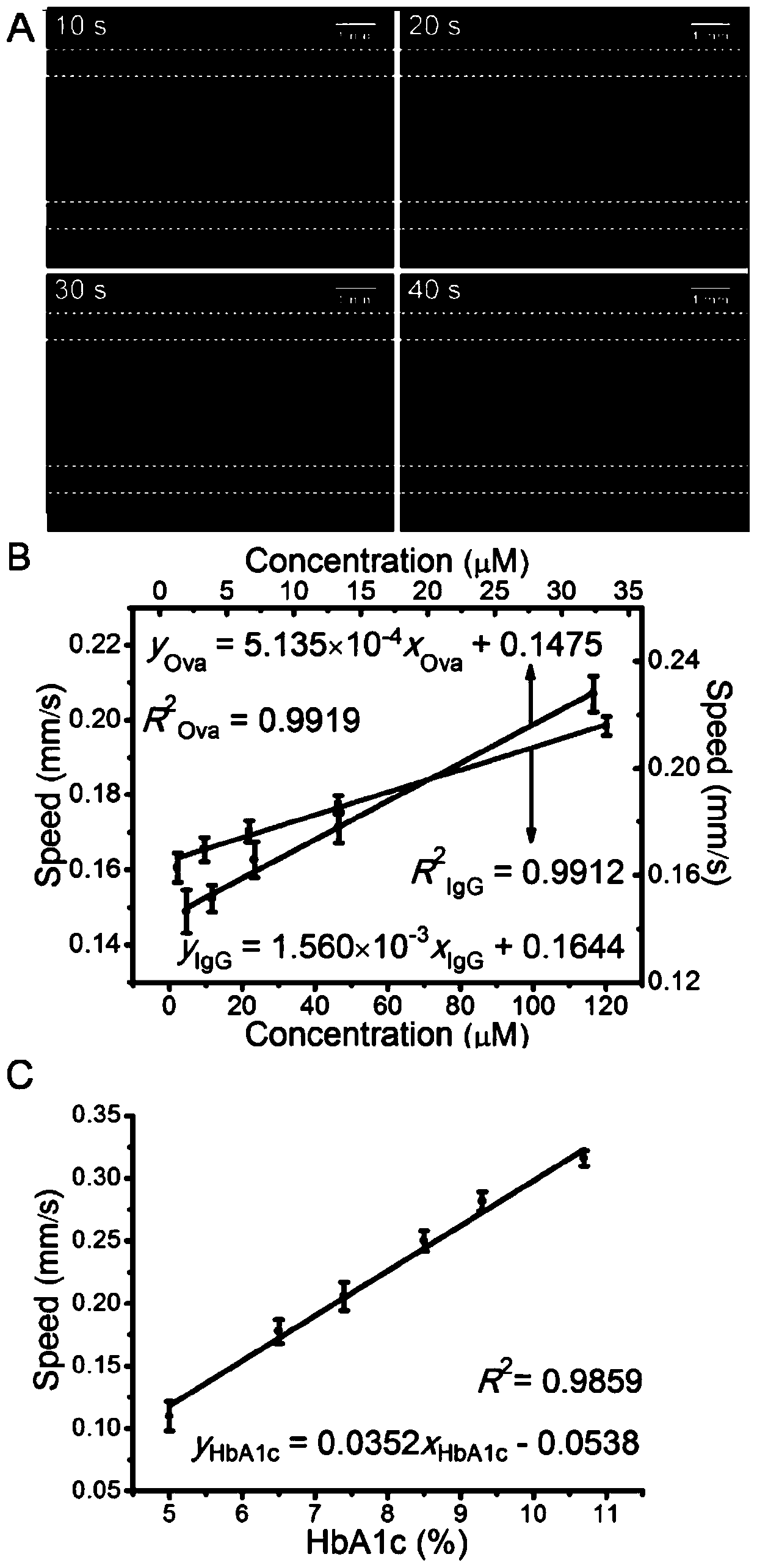
[0034] In this embodiment, taking the detection of immunoglobulin G (hereinafter referred to as IgG) as an example, the FSS-CSE test is first performed on the prepared IgG standard samples of various concentrations to obtain the fluorescence sensing speeds corresponding to the standard samples of various concentrations, so that A standard curve of fluorescence sensing speed and protein concentration was constructed ( image 3 -B). After constructing the standard curve, the same method can be used to measure the IgG of unknown concentration. After measuring the fluorescence sensing speed of IgG of unknown concentration, the concentration value can be calculated according to the fluorescence sensing speed and the standard curve.
[0035] This embodiment specifically includes the following steps:
[0036] In the first step, take the quantitative determination of ovalbumin concentration as an example. In this embodiment, 5 concentration points are selected, which are 0.2, 0.5, 1...
Embodiment 2
[0047] In this embodiment, the ovalbumin in Example 1 is replaced by glycosylated hemoglobin (HbA1c) related to diabetes detection, and a standard curve can be obtained similarly, such as image 3 C shows. The content of glycosylated hemoglobin in human blood can be obtained using the same method as above.
[0048] The gel is also a polyacrylamide gel, and its concentration, cross-linking degree and components are consistent with those in the ovalbumin FSS-CSE test.
[0049] Both the background electrolyte solution and the electrode buffer are 20 mM pH 7.4 phosphate buffer solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com