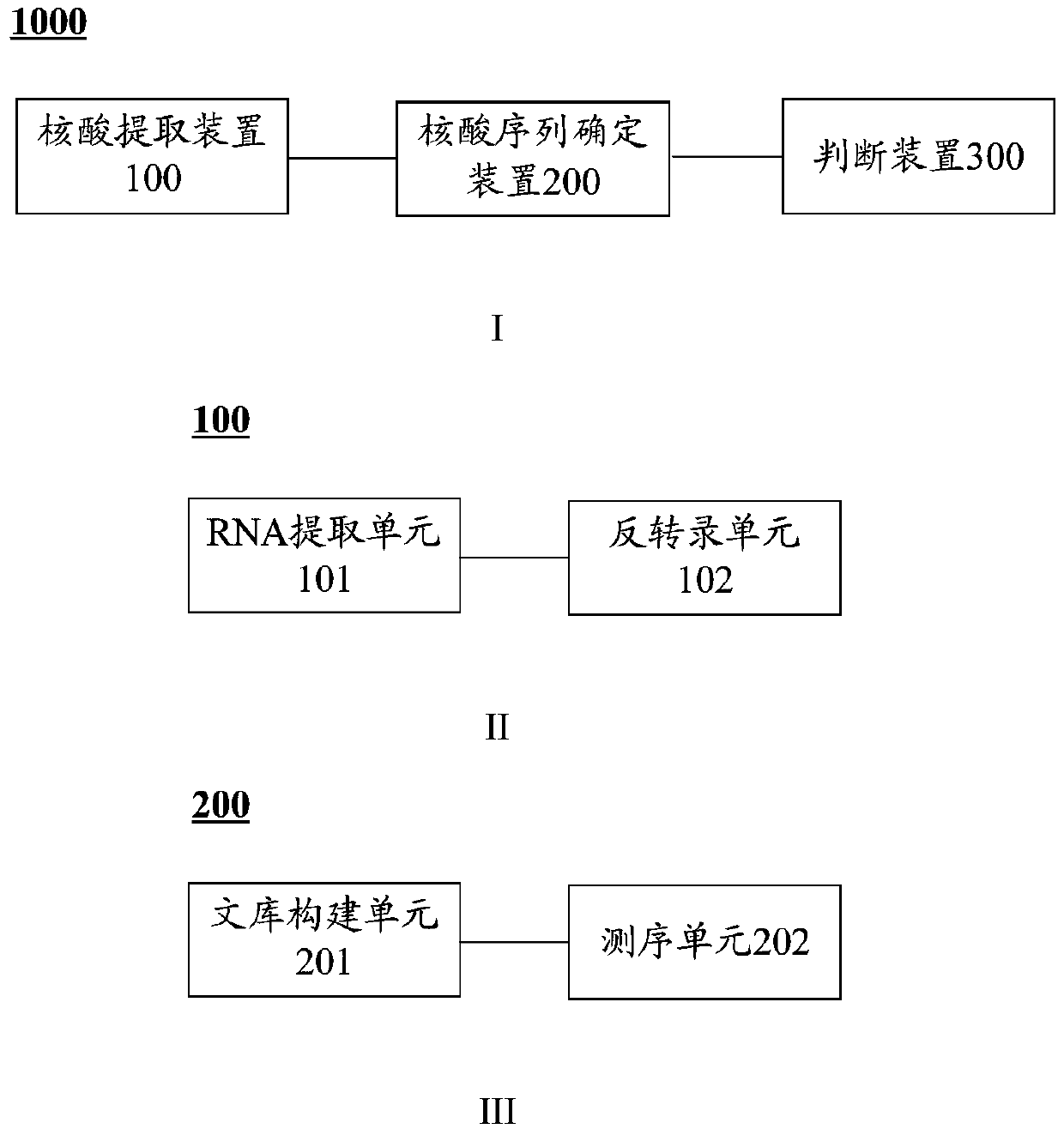
cyp4v2 gene mutant and its application
A CYP4V2, mutant technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve problems that need to be deepened
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Determination of pathogenic mutations in primary crystalline retinal degeneration (BCD)
[0064] 1. Sample collection
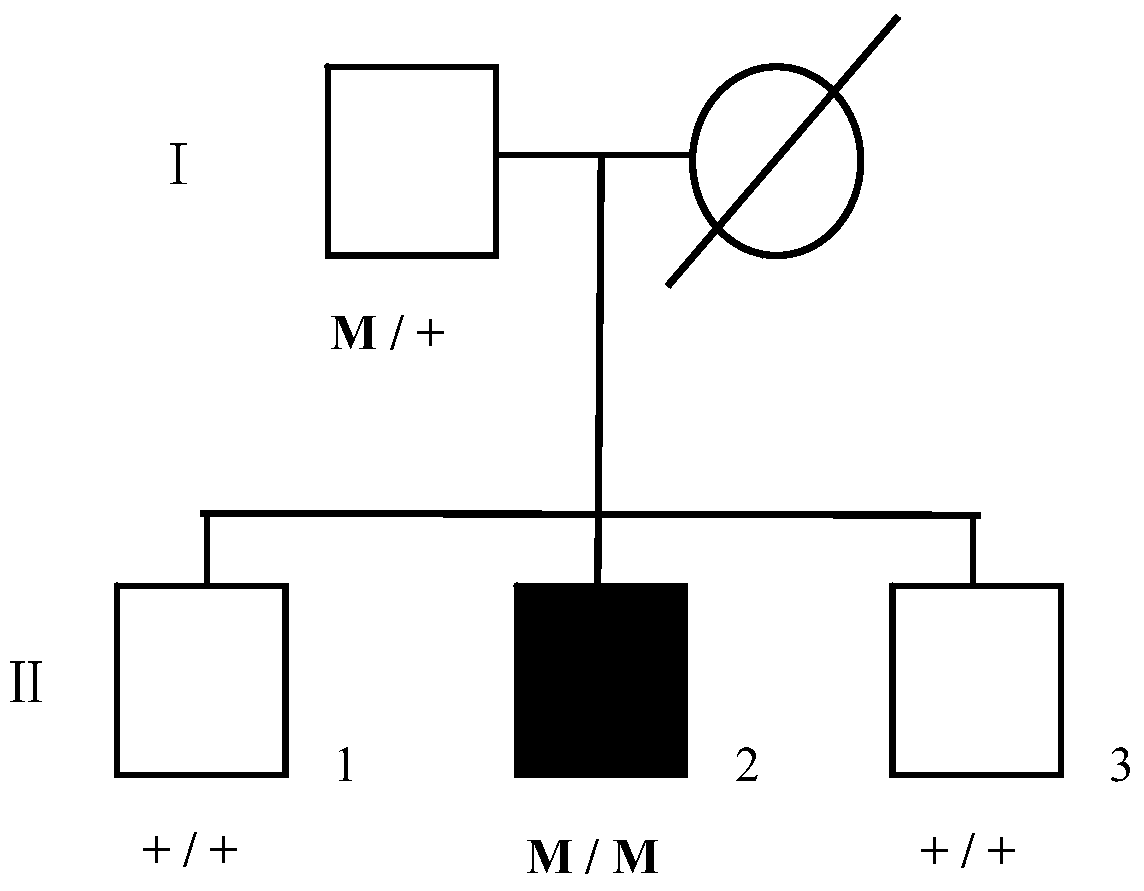
[0065] In 2010, the inventor collected a case of BCD patient in Chongqing. There were 4 family members of this patient, including 1 patient and 3 normal members. A total of 4 people participated in the research of the present invention, including 1 patient, 3 normal members (father, elder brother and younger brother), and all family members participating in the research of the present invention signed the informed consent. The patient's pedigree figure 2 As shown, where □ indicates a normal male, ■ indicates a male patient, Denotes a deceased normal female, M denotes a mutation, and + denotes a wild type.
[0066] In this family, the patient developed night blindness at the age of 49, and the fundus examination showed typical crystal-like retinal degeneration changes. image 3 A fundus image of this BCD patient is shown. Depend on im...
Embodiment 2
[0077] Example 2: Sanger method sequencing verification
[0078] Primers were designed for the sequence of CYP4V2 gene (exon 1-11), and the related sequence was obtained by PCR amplification, product purification and sequencing. The specific operation is as follows:
[0079] 1. DNA extraction
[0080] Collection of probands in the BCD family ( figure 2 Members represented by middle ■) peripheral blood, using the conventional phenol-chloroform method to extract the genomic DNA in the peripheral blood leukocytes, using a spectrophotometer to measure the concentration and purity of the DNA, and the OD of the genomic DNA of each sample obtained 260 / OD 280 They are all located between 1.7-2.0, the concentration is not less than 200 ng / microliter, and the total amount is not less than 30 micrograms.
[0081] 2. Primer Design
[0082] The PCR reaction primers were designed with reference to the human genome sequence, as shown in Table 1 below:
[0083] Table 1
[0084]
...
Embodiment 3
[0094] Embodiment 3: Sanger method sequencing verification
[0095]The CYP4V2 gene of all family members (including patients and normal people in the family) and 100 normal people outside the family in the BCD patient family described in Example 1 were detected: using the exons of the CYP4V2 gene in Example 2 1. Design the primers, obtain the relevant sequence of the mutation site by PCR amplification, product purification and sequencing, and verify the correlation between the CYP4V2 gene and the mutation and BCD according to whether the sequence determination results belong to the mutant type or the wild type.
[0096] The specific method steps are as follows:
[0097] 1. DNA extraction
[0098] According to the method for extracting DNA described in Example 2, the genomic DNA in the peripheral venous blood of the subject was extracted and prepared respectively for future use.
[0099] 2. PCR reaction
[0100] Using the primers and PCR conditions designed for exon 1 of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com