An orally administrable hypoglycemic polypeptide and its preparation method and application
A technology for lowering blood sugar and polypeptide sequence, applied in the field of medicine and biology, can solve problems such as difficulty in absorption, and achieve the effect of improving anti-enzymatic hydrolysis ability and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
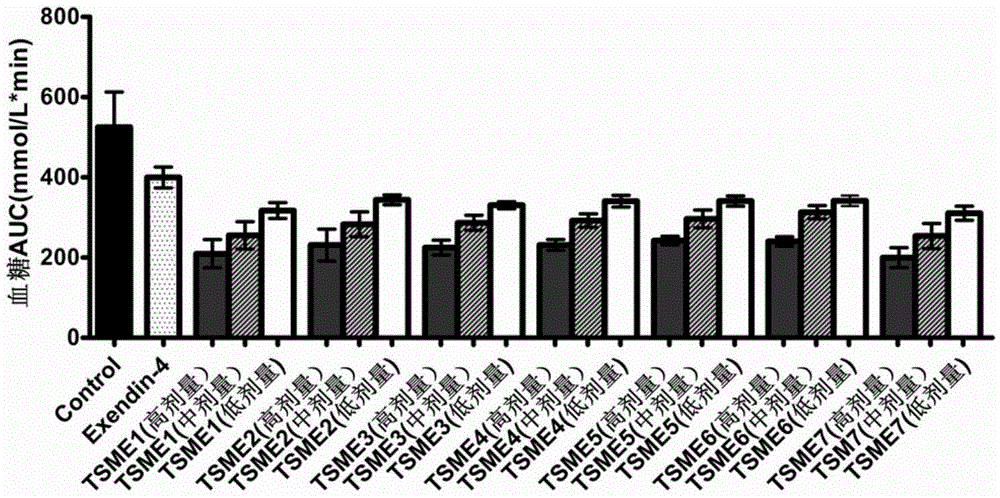
[0050] TSME1-7 polypeptide oral hypoglycemic activity determination:
[0051] The oral hypoglycemic activity of TSME1-7 was evaluated by intraperitoneal glucose tolerance test (IPGTT):
[0052] Step 1: Prepare 1 mg / mL Exendin-4 solution and 1, 0.1, 0.01 mg / mL TSME1-7 solution with PBS solution. Then prepare a solution containing 25% PBS / Exendin-4 / TSME1-7, 25% propylene glycol and 50% NaHCO 3 Solution (3% concentration) mixture.
[0053] Step 2: 138 male Balb / C mice were selected and randomly divided into 23 groups, 6 in each group. Groups and doses are as follows: blank control group (group 1) (PBS solution); positive control group (group 1) (Exendin-4 prototype molecule 500nmol / kg); TSME1-7 (group 3) in three doses (5nmol / kg). kg, 50nmol / kg, 500nmol / kg).
[0054] Step 3: Fasting the mice for 18 hours. Then blood was taken from the tail vein, and the blood glucose concentration was measured with a blood glucose meter as a 0-point blank blood sample.
[0055] Step 4: intr...
Embodiment 2
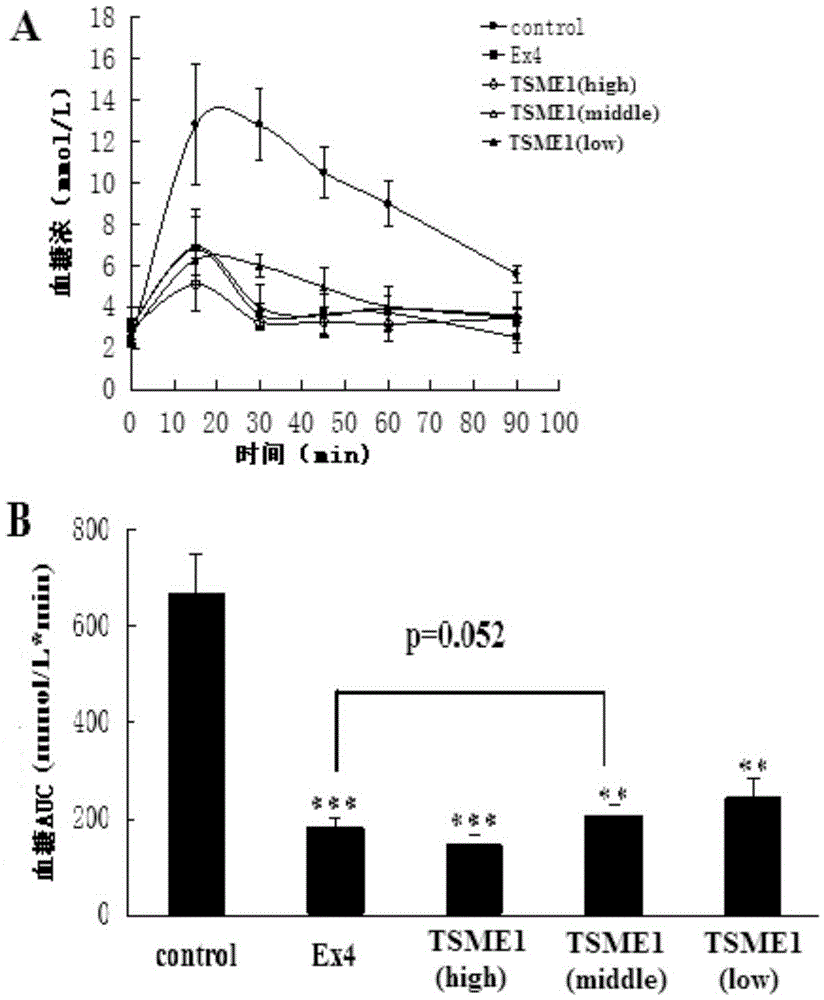
[0058] TSME1 biological activity detection in vivo:
[0059] The hypoglycemic activity of TSME was evaluated by intraperitoneal glucose tolerance test (IPGTT):
[0060] Step 1: Select 30 male Balb / C mice and randomly divide them into 5 groups with 6 mice in each group. The groups and doses are as follows: blank control group (group 1) (PBS solution (20mmol / L)); positive control group (group 1) (Exendin-4 prototype molecule 9nmol / kg); TSME1 (group 3) three doses (3nmol / kg, 9nmol / kg, 27nmol / kg).
[0061] Step 2: Fasting the mice for 18 hours. Then blood was taken from the tail vein, and the blood glucose concentration was measured with a blood glucose meter as a 0-point blank blood sample.
[0062] The third step: intraperitoneal injection to mice in each group (see the first step for the dosage).
[0063] Step 4: intraperitoneally inject glucose solution 30 minutes after the administration (the dosage is the same as in Example 1). Then blood was taken from the tail vein at...
Embodiment 3
[0065] Determination of TSME1 resistance to enzymatic hydrolysis:
[0066] Step 1: prepare 2 mmol / L trypsin solution with PBS solution with pH=6.5. Use after incubating at 37°C for 30 minutes. At the same time, a 200ug / mL solution of Exendin-4 and TSME1 was prepared.
[0067] Step 2: Mix 20 uL of Exendin-4 and TSME1 solution with 20 uL of enzyme solution, and then stop the reaction with 100 uL of 1% TFA solution at predetermined time points (0, 5, 10, 15, 20). After centrifugation at 12000rpm for 5min, the supernatant was detected by RP-HPLC.
[0068] Step 3: Calculate the residual rate. The residual rate at 0 point is 100%. The ratio of the peak area of each point to the peak area of 0 point is the residual rate of each point. see attached results image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com