Blood-borne miRNA as surrogate markers of drug efficacy for cardiac conditions
A technology for efficacy and heart disease, applied in the direction of drug combination, cardiovascular system diseases, active ingredients of heterocyclic compounds, etc., can solve problems such as hindering treatment, lack of molecular level diagnostic technology, and difficulty in determining the cause of heart failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1: Blood-borne miRNAs as Surrogate Markers of Drugs for Cardiac Disorders
[0156] The importance of miRNAs for cardiac function and dysfunction suggests an opportunity to therapeutically exploit the biology of miRNAs in the setting of cardiac disease. Single-stranded RNA oligonucleotides have been shown to be effective for inactivating miRNAs in vivo by complementary base pairing (Elmen, J. et al. (2008) Nature 452, 896-899; Elmen, J. et al. (2008) Nucleic Acids Res 36 , 1153-1162; Krutzfeldt, J. et al. (2007) Nucleic Acids Res35, 2885-2892; Krutzfeldt, J. et al. (2005) Nature 438, 685-689; Lanford, R.E. et al. (2010) Science 327, 198-201) , and represent a potentially effective means of inactivating pathological miRNAs.
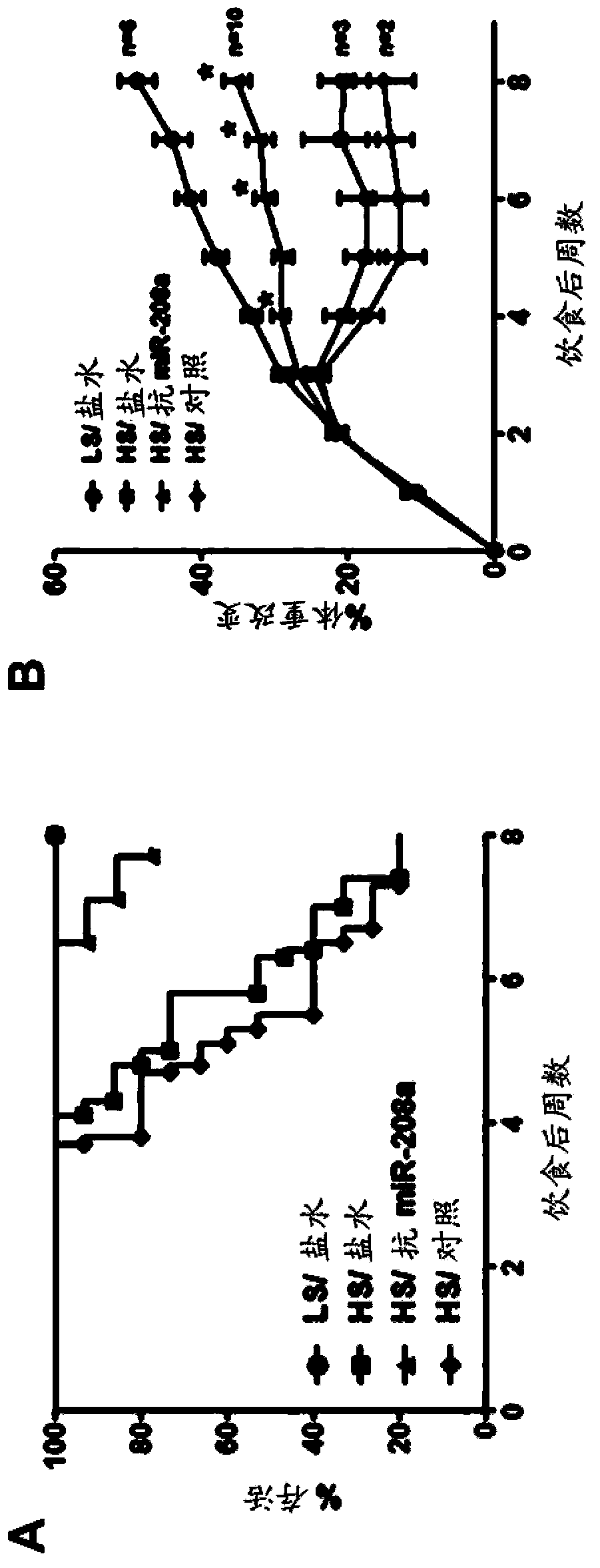
[0157] This example describes the therapeutic efficacy of antisense oligonucleotides targeting miR-208a in a model of heart failure. Specifically, we show that systemic delivery of locked nucleotide (LNA)-modified antisense oligonucleotides...
Embodiment 2
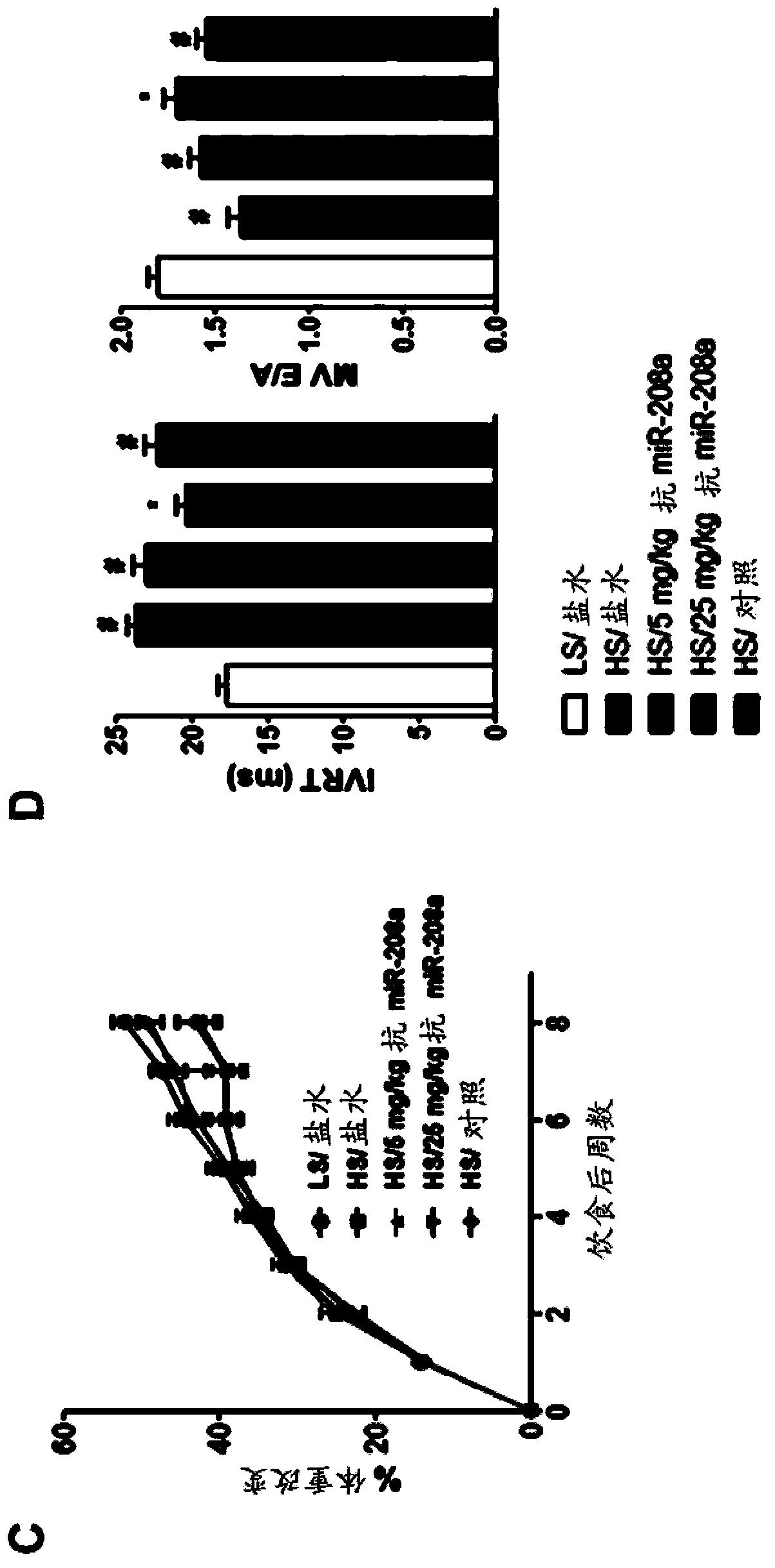
[0171] Example 2: Plasma miRNAs are biomarkers of disease progression in heart failure and therapeutic efficacy of anti-miR-208 oligonucleotides.
[0172] To assess plasma biomarkers over time, a surgically implanted catheter was used to draw blood non-invasively every two weeks in the Dahl salt-sensitive rat model of congestive heart failure described in Example 1 . Changes in plasma miRNA biomarkers with disease progression were assessed following treatment with an LNA-modified antisense oligonucleotide (M-10101; SEQ ID NO: 12) targeting miR-208a. The following experimental groups were tested:
[0173] Group
Strain
diet
deal with
group 1
Dahl SS
regular feed
subcutaneous saline
group 2
Dahl SS
4% NaCl diet
subcutaneous saline
group 3
Dahl SS
4% NaCl diet
Subcutaneous M-10101 anti-miR (25mg / kg)
group 4
Dahl SS
4% NaCl diet
Subcutaneous M-10591 control (25mg / kg)
[0174]...
Embodiment 3
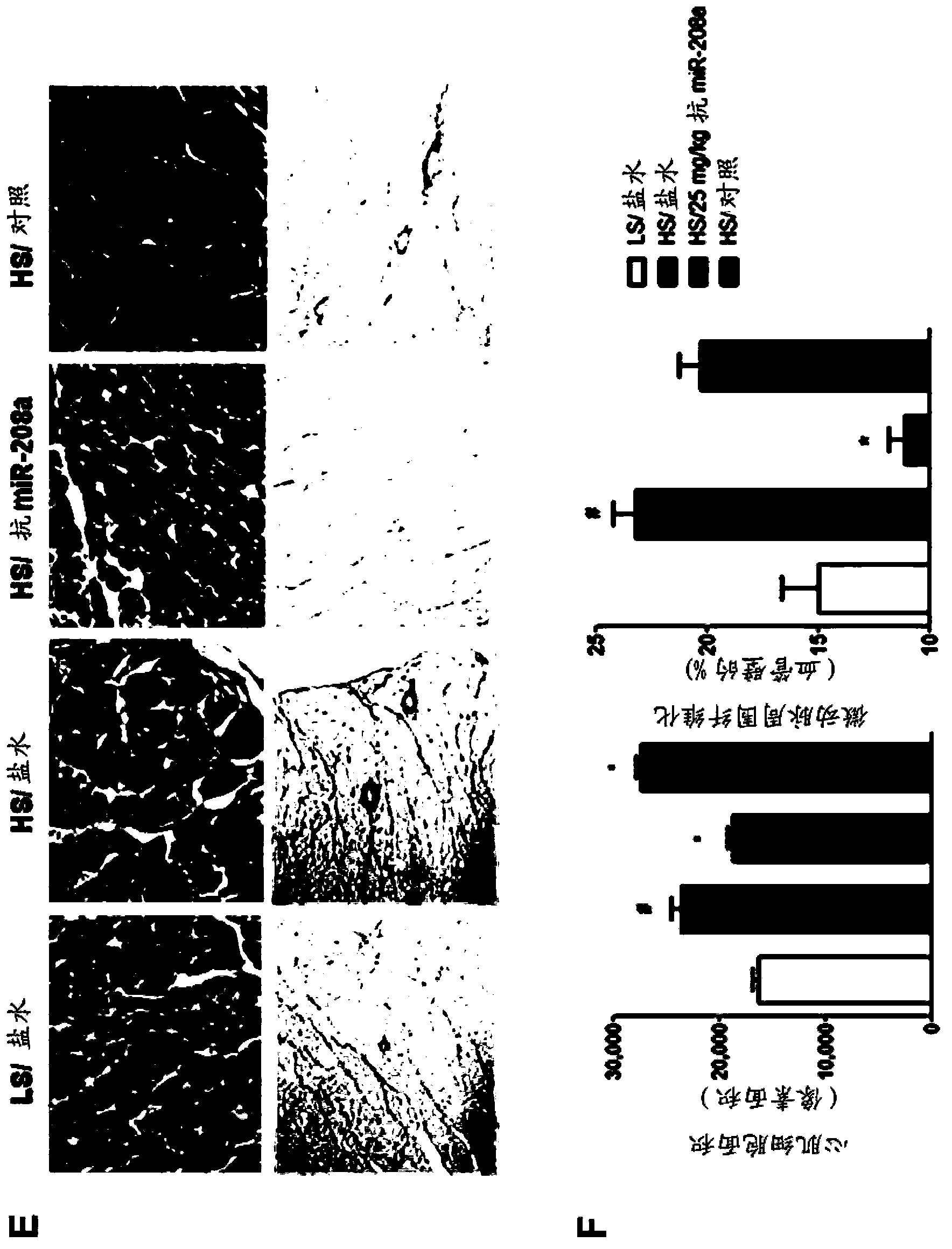
[0196] Example 3: Anti-miR Treatment and Captopril Treatment Affect Circulating miRs
[0197] The effect of different therapeutic approaches on plasma miRNA levels was assessed using the Dahl salt-sensitive rat model of congestive heart failure (see Example 1). Specifically, anti-miR-208a oligonucleotides containing LNA (M-10101), captopril (an angiotensin-converting enzyme (ACE) inhibitor) or a combination of both (M-10101+ Captopril) treated rats.
[0198] The following experimental groups were evaluated:
[0199] Group
Strain
N
diet
deal with
group 1
Dahl SS
6
regular food
Saline sc / water po
group 2
Dahl SS
10
6% NaCl diet
Saline sc / water po
group 3
Dahl SS
9
6% NaCl diet
M-10101(25mg / kg) sc / water po
group 4
Dahl SS
9
6% NaCl diet
Saline sc / captopril (1.5mg / kg) po
Group 5
Dahl SS
9
6% NaCl diet
Saline sc / captopril (15mg / kg) po
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com