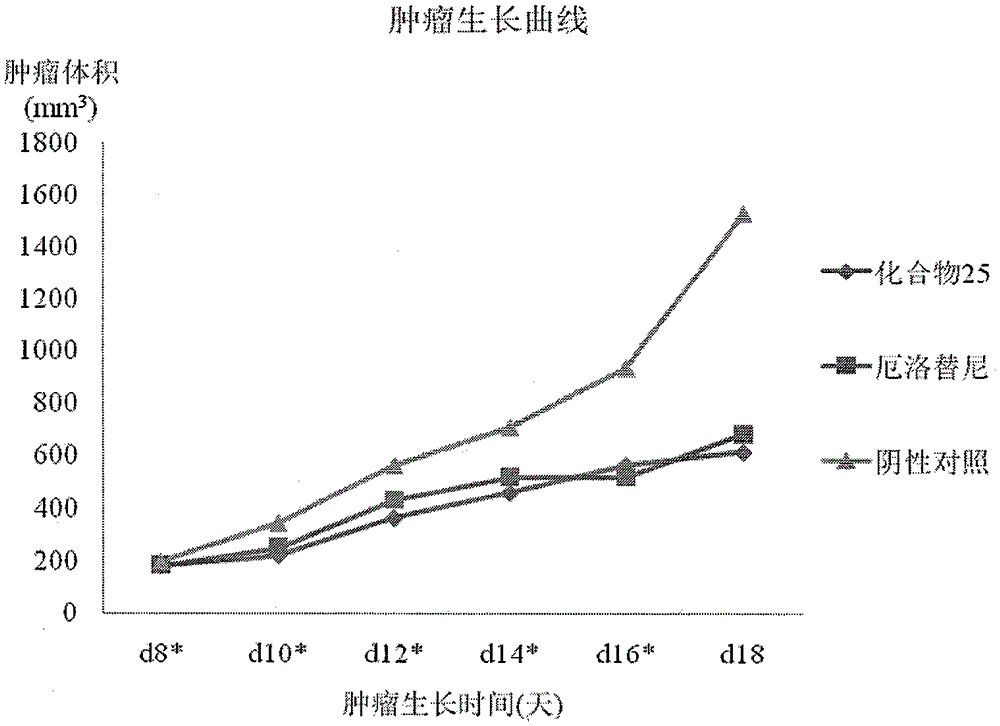
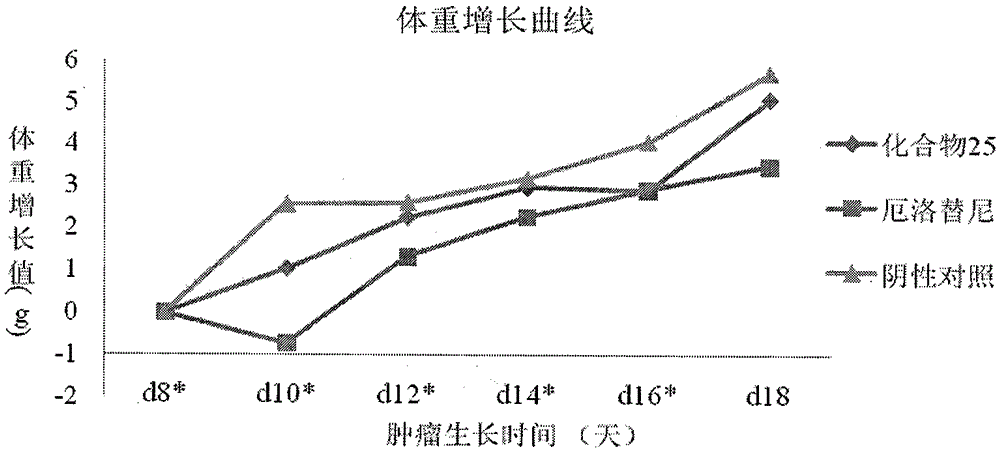
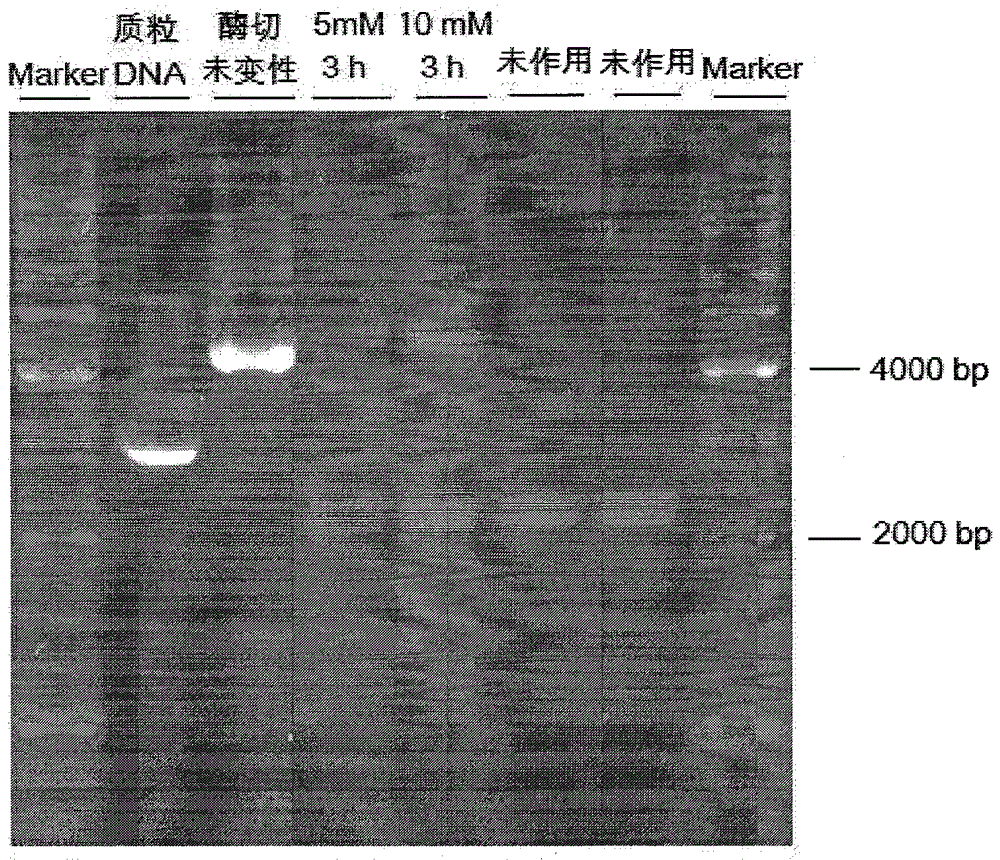
Novel quinazoline mustard compound and its preparation method and tumor treatment application
A quinazoline mustard, tumor treatment technology, applied in anti-tumor drugs, organic chemistry, drug combination and other directions, can solve the problem of not fully excavating the potential of anti-cancer drugs, and achieve good anti-cancer activity and good anti-tumor effect, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0093] According to the following steps, the substituents R1=meta nitrogen mustard, R2=methoxyethoxy, R3=methoxyethoxy compounds in formula A are prepared.
[0094] 1.1N, the synthesis of N-bis(2-hydroxyethyl)-3-nitroaniline
[0095] Take 5.52 g (0.04 mol) of 3-nitroaniline, add it to 80 mL of 25% acetic acid aqueous solution, and add 10 mL of ethylene oxide dropwise under ice-cooling. Warm up to room temperature, stir for 72 hours, filter with suction, wash the filter cake with water, and dry to obtain 8.02 g of a yellow-orange solid, with a yield of 88%. mp94-96℃, 1 HNMR (400MHz, CDCl 3 )δ7.53 (d, J=8.0Hz, 1H, ArH), 7.48 (s, 1H, ArH), 7.33 (t, J=8.2Hz, 1H, ArH), 6.97 (dd, J=8.4, 2.2Hz , 1H, ArH), 3.89 (t, J=4.8Hz, 4H, -NCH 2 CH2-), 3.65(t, J=4.8Hz, 4H, -CH 2 CH 2 OH). 13 CNMR (100MHz, CDCl 3 )δ149.43, 148.63, 129.84, 118.10, 111.34, 106.48, 60.35, 55.06.MS (ESI + ) m / z: 227.3 (M+H + ).IR(KBrpellet, cm -1 ): 3265, 3173, 2959, 2863, 1618, 1565, 1525, 1486, 1341, 129...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com