Novel application of caffeoylquinic acid compound
A dicaffeoyl and drug technology, applied in the field of caffeoylquinic acid compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
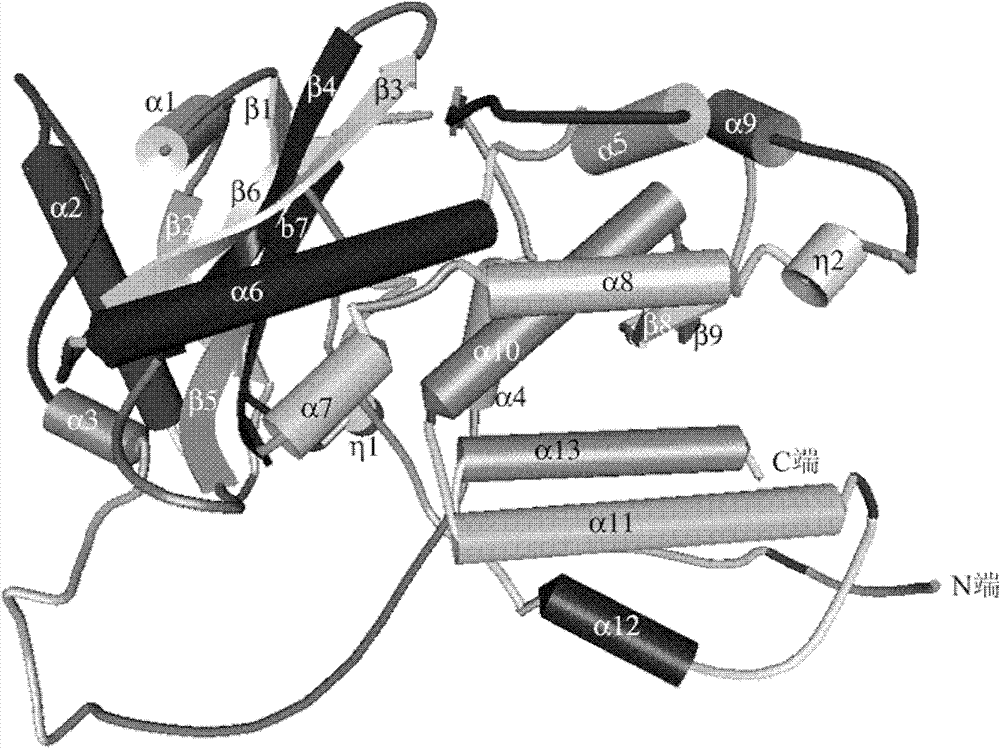
Image
Examples
Embodiment 1
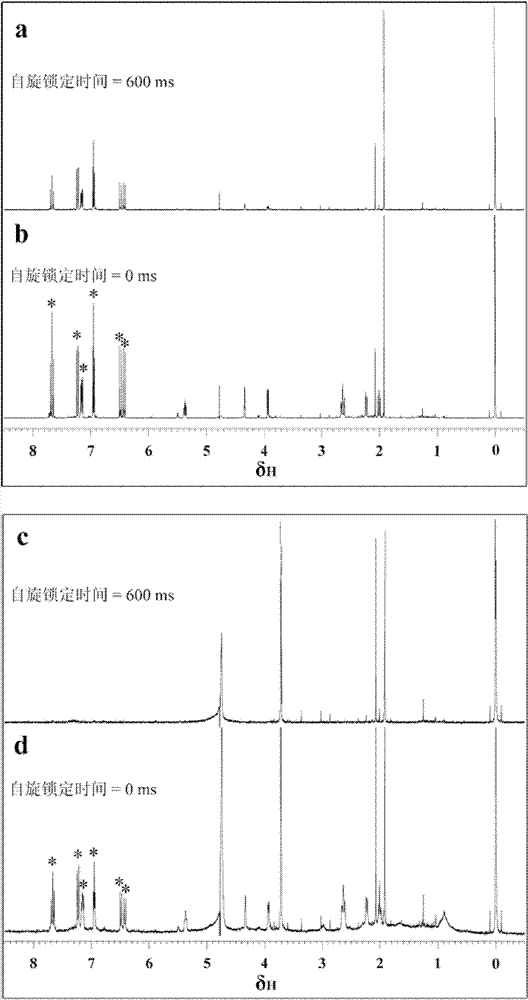
[0064] Embodiment 1, determine caffeoylquinic acid compound and PA by NMR experiment C Interaction dissociation constant K d
[0065] The NMR experiment was completed with a Bruker AVANCE 600 spectrometer (equipped with a 5mm BBI probe and provided with a gradient in the z direction), and the experimental operation and data processing were completed using a TOPSPIN (Bruker, version 2.1) workstation. All NMR experiments were performed at 298.2K (Bruker, B3000 temperature control unit). Relaxation edited NMR spectra using [D / presaturation-90 x -(Δ-180 y -Δ) n -acquire] pulse sequence, wherein the Carr-Purcell-Meiboom-Gill (CPMG) sequence is used for spin-lock (spin-lock), and a pre-saturation pulse is used to suppress the water peak. For all relaxation editing experiments, D = 3s, P 90 (90°pulse) is set according to different samples, Δ=1.5ms, 2×n×Δ=total spin-locking time, the number of spin-locking cycles n is set according to different experiments, the number of samplin...
Embodiment 2
[0122] Embodiment 2, RNA influenza virus polymerase activity test
[0123] The RNA influenza virus polymerase activity was tested by the ApG primer extension method reported by T. Deng et al. in J. Virol. 80 (2006) 2337-2348. The specific method is as follows: in 5mM MgCl 2 , 5mM small molecules (caffeoylquinic acid compounds), 5mM dithiothreitol, 1mM adenosine triphosphate (ATP), 0.5mM uridine triphosphate (UTP), 0.5mM cytidine triphosphate (CTP), 0.1μM[ α- 32P] guanosine triphosphate (GTP 3000Ci / mmol), and 2U / μl RNasin (Promega Company) in the presence of 2.5 μL H5N1 avian virus RNA polymerase (3P) and 0.7 μM template virus RNA promoter (equal molar 5′- A mixture of end vRNA 5′-AGUAGAAACAAGGCC-3′ and 3′-endvRNA 5′-GGCCUGCUUUUGCU-3′) was performed in a 5 μL reaction. 0.5mM ApG primer sequence (Sigma company) was added to the reaction system and incubated at 30°C for 1 hour. After adding 5 μL of 2× formamide / bromophenol blue / EDTA loading buffer, the mixture was heated at 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com