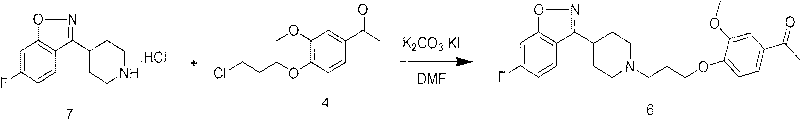Method for synthesizing Iloperidone
A technology of iloperidone and a synthesis method, which is applied in the field of synthesis of iloperidone, can solve the problems of low water solubility and removal of dimers, and achieves the effects of simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
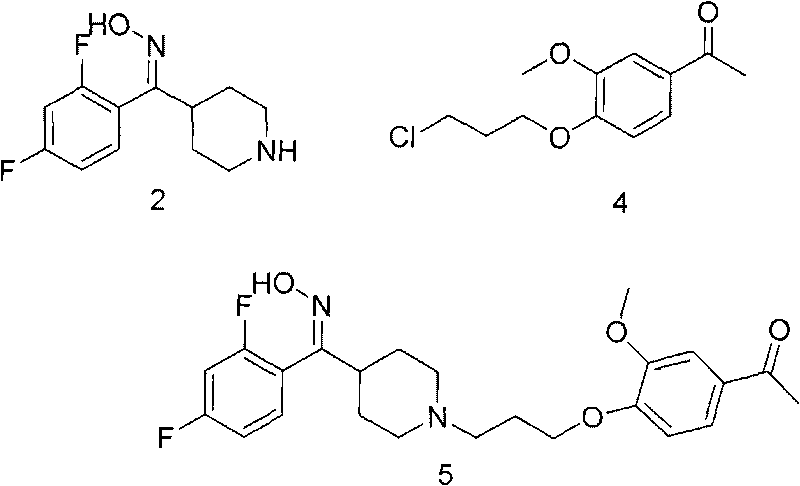
[0029] Preparation of compound (2) hydrochloride
[0030] Under nitrogen protection, 2,4-difluorophenyl-4-piperidinyl ketone hydrochloride (1) (60.0g, 0.25mol) and hydroxylamine hydrochloride (60g, 0.86mol) were added to a 1L reaction flask, Add 600ml of absolute ethanol, add triethylamine (75.0ml, 0.54mol) dropwise under stirring, control the temperature below 30°C, heat to reflux after dropping, react for 5 hours, and when the reaction is over, filter out the solid while it is hot, and dry it to obtain Compound (2) hydrochloride 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride 44.0 g, yield 80.0%.
[0031] Preparation of compound (2)
[0032] Dissolve 25.0 g of 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride in 100 ml of water, heat to 50 ° C, neutralize to PH = 10 with 10% sodium hydroxide solution, and cool After reaching room temperature, the solid was obtained by suction filtration, and dried to obtain compound (2) 2,4-difluorophenyl(4-piperi...
Embodiment 2
[0042] Preparation of compound (5)
[0043]Under nitrogen protection, 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride (20.0 g, 72.4 mmol) was added to a 250 ml reaction flask, 150 ml of acetonitrile was added, and sodium bicarbonate ( 14.0g, 166.7mmol), sodium iodide 0.8g, stirred for 10 minutes, 1-(4-(3-chloropropyl)-3-methoxy)acetophenone (17.6g, 72.5mmol) was added to the reaction In the bottle, heated to reflux at 100°C, kept stirring at reflux for 20 hours, cooled to room temperature, cooled to 0°C in an ice bath, precipitated solid, filtered with suction, washed the solid with 50ml of water, and dried to obtain 28.1g of compound (5). 87.0%.
[0044] Preparation of compound (6) iloperidone
[0045] Under nitrogen protection, compound 5 (10.0g, 22.4mmol) was added to a 250ml reaction flask, then 100ml of acetone was added, potassium hydroxide (2.51g, 44.8mmol) was added and heated to reflux at 60°C, kept stirring at reflux for 10 hours, cooled To room temp...
Embodiment 3
[0047] Preparation of compound (5)
[0048] Under the protection of nitrogen, 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride (10.0g, 36.2mmol) was added to a 250ml reaction flask, and N,N-dimethylformaldehyde was added Amide 100ml, add potassium hydroxide (6.1g, 108.6mmol), stir for 10 minutes, add 1-(4-(3-chloropropyl)-3-methoxy)acetophenone (8.8g, 36.2mmol) Put it into a reaction bottle, heat it to 100°C, keep the temperature and stir for 15 hours, cool down to room temperature, cool to 0°C in an ice bath, precipitate a solid, filter it with suction, wash the solid with 30ml of water, and dry to obtain compound (5) 13.49, yield 83.0%.
[0049] Preparation of compound (6) iloperidone
[0050] Under the protection of nitrogen, compound 5 (20.0g, 22.4mmol) was added to a 250ml reaction flask, then 100ml of tetrahydrofuran was added, potassium hydroxide (3.779, 67.2mmol) was added and heated to reflux at 80°C, kept stirring at reflux for 8 hours, and cooled to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com